Pathogenic BRCA1 mutations disrupt allosteric control by BARD1
Pathogenic BRCA1 mutations disrupt allosteric control by BARD1
Bhattacharjee, A.; Bowman, G.
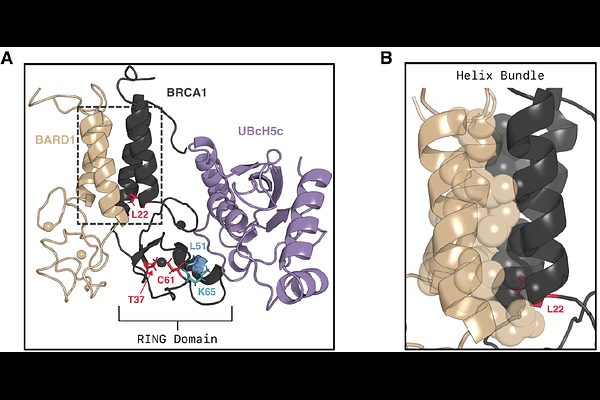
AbstractMechanistic insight into biophysical perturbations caused by pathogenic missense mutations is highly valuable information for the rational design of therapeutics. For hereditary breast and ovarian cancer, multiple pathogenic mutations in the N-terminal domain of BRCA1 have been reported in patients. How exactly these mutations disrupt the catalytic activity of BRCA1, and thereby lead to oncogenesis, is unknown. Here, we posit that the mechanism of pathogenesis is tied to how binding of BARD1 activates BRCA1 for E3 ligase activity. We use atomistic molecular dynamics simulations and Markov state modeling to uncover how BARD1 selects for active conformational states of BRCA1. We show that the helix bundle, where BARD1 binds, is allosterically coupled to the E2 interface. Furthermore, we show that BARD1 selects for conformational states that are pre-organized for E3 activity. Lastly, we show that pathogenic mutations allosterically destabilize active states, whereas hyperactive mutations constitutively increase their likelihood. These results provide a concrete strategy supported by mechanistic insight for the design of restorative small molecules targeting BRCA1.