Interprotomer Crosstalk in Mosaic Viral Glycoprotein Trimers Provides Insight into Polyvalent Immunogen Co-assembly
Interprotomer Crosstalk in Mosaic Viral Glycoprotein Trimers Provides Insight into Polyvalent Immunogen Co-assembly
Chen, C.; Lovendahl, K.; Overbaugh, J.; Lee, K. K.
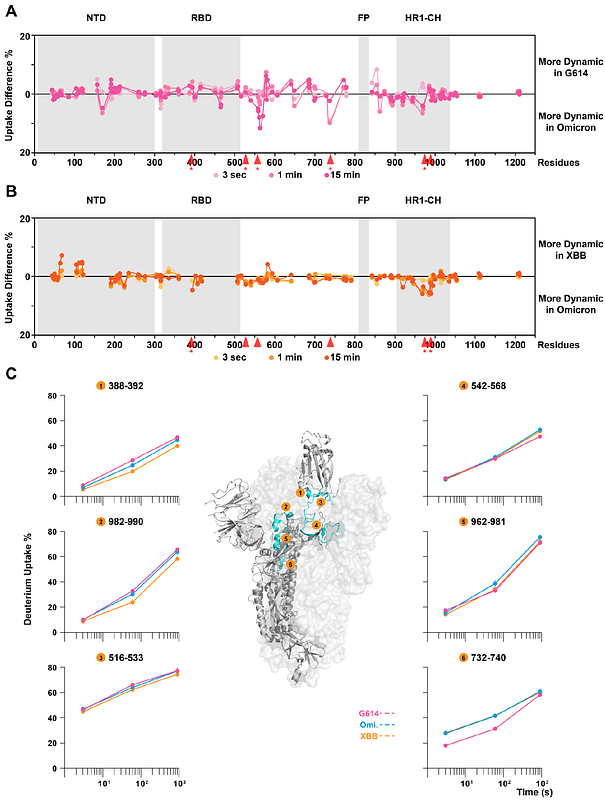
AbstractSARS-CoV-2 variants have demonstrated the ability to evade immune responses, leading to waves of infection throughout the pandemic. In response, bivalent mRNA vaccines, encoding the original Wuhan-Hu-1 and emerging variants, were developed to display both spike antigens. To date, it has not been determined whether co-transfection and co-translation of different SARS-CoV-2 variants results in co-assembly of mosaic heterotrimer antigens and how this may affect trimer stability, dynamics, and antigenicity. Understanding whether such mosaic heterotrimers can form and their implications for antigen structure can provide important information to guide future polyvalent vaccine design where multiple variants of an antigen are co-formulated. To investigate this, we purified mosaic spike assemblies of both genetically close (Omicron BA.2 and XBB) and distant (Omicron BA.2 and Wuhan-Hu-1 G614) strains. We found that the stability and integrity of mosaic spike trimers were maintained without misfolding or aggregation. Glycosylation profiles likewise were preserved relative to the homotrimer counterparts. Hydrogen/deuterium-exchange mass spectrometry and biolayer-interferometry were used to investigate the mosaic spike dynamics and any impact on epitope presentation and receptor binding. The Omicron-XBB heterotrimer, sharing a common fusion subunit sequence, retained protomer-specific dynamics similar to the corresponding homotrimers in antigenically important regions. The Omicron-G614 heterotrimer, co-assembling from protomers of divergent fusion subunit sequences, likewise showed overall similar dynamics and conformations in the receptor-binding subunit compared to the homotrimers. However, the incorporation of the Wuhan-Hu-1 G614 protomer led to a stabilizing effect on the relatively unstable Omicron fusion subunit in the heterotrimer. These findings reveal structural dynamic crosstalk in mosaic trimers, suggesting a potential for enhanced immunogen display and important considerations to be aware of in the use of polyvalent nucleic acid vaccines.