Multi-omics integration uncovers host-microbiota crosstalk underlying sexual differentiation in the shortfin eel Anguilla bicolor pacifica
Multi-omics integration uncovers host-microbiota crosstalk underlying sexual differentiation in the shortfin eel Anguilla bicolor pacifica
Brandon-Mong, G.-J.; Lee, T.-H.; Chen, Y.-L.; Hsiao, T.-H.; Wu, G.-L.; Lai, Y.-L.; Weng, C.-Y.; Gicana, R. G.; Yen, Y.-T.; Xu, Y.-F.; Wang, T.-Y.; Chiang, Y.-R.
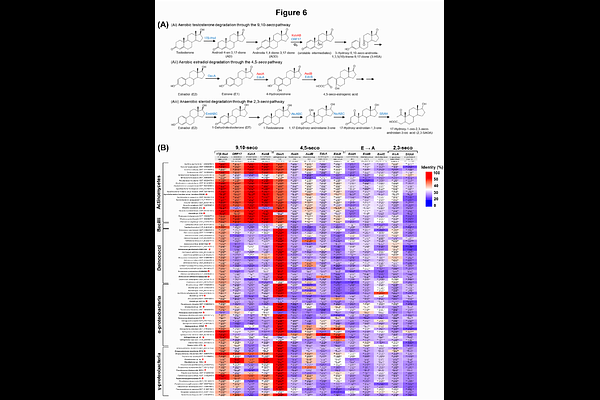
AbstractThe global decline in anguillid eel populations has intensified interest in understanding their biology for conservation and aquaculture. While host-gut microbiota interactions are well-characterized in homeotherms, these relationships remain poorly understood in poikilotherms during sexual differentiation. We examined gut microbiota dynamics across developmental stages in the shortfin eel Anguilla bicolor pacifica, which exhibits early sexual differentiation and a relatively short life cycle. Glass eels were cultivated in controlled freshwater conditions for three years, with sampling at key stages: glass eel, elver, sex-undetermined eel, and sex-determined eel. Full-length 16S rRNA gene sequencing revealed significant compositional shifts during development, with higher bacterial richness in adults versus younger eels. Early stages were dominated by Pseudomonadota, while sex-determined adults showed increased Deinococcota abundance. Network analysis identified Deinococcus, Sphingomonas, and Variovorax as key genera in sex-determined eels, with positive correlations between anti-Mullerian hormone gene expression and these taxa. We isolated 66 gut bacterial strains capable of metabolizing sex hormones under microaerobic conditions. These isolates, representing 22 genera across four phyla, demonstrated diverse metabolic capabilities from partial oxidation to complete steroid mineralization. Multiple strains achieved complete estradiol degradation as single isolates, a rare metabolic capability of environmental microorganisms. Comparative genomic analysis revealed widespread steroid-metabolizing genes, with Deinococcus species showing previously unreported hormone degradation capabilities. Our multi-omics analysis demonstrates that gut microbiota composition and function are intimately linked to eel sexual development, suggesting bidirectional host-microbe interactions influencing reproductive physiology. These findings advance understanding of host-microbiota interactions in aquatic vertebrates and provide implications for eel aquaculture and conservation.