A single valine to leucine switch disrupts Plasmodium falciparum AP2-G DNA binding and reveals the role of GDV1 in ap2-g activation.
A single valine to leucine switch disrupts Plasmodium falciparum AP2-G DNA binding and reveals the role of GDV1 in ap2-g activation.
Prajapati, S. K.; Dong, J. X.; Morahan, B. J.; Dotrang, T.; Barbeau, M. C.; Williams, A. E.; Hupalo, D.; Wilkerson, M. D.; Dalgard, C. L.; Kafsack, B. F. C.; Llinas, M.; Williamson, K. C.
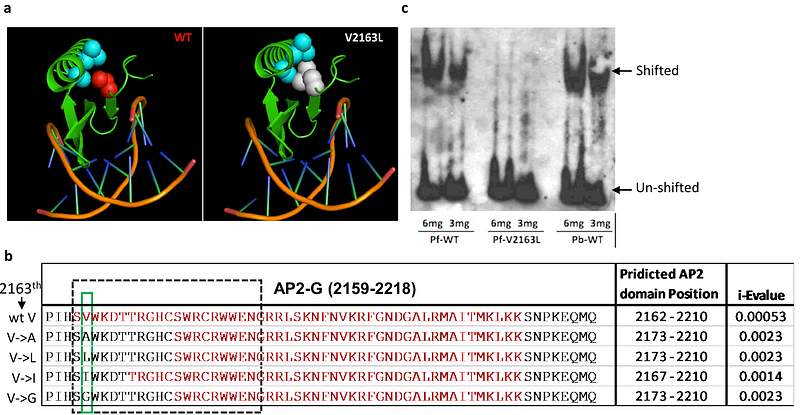
AbstractSexual commitment in Plasmodium parasites is essential for malaria transmission, yet much remains unknown about the underlying signaling events initiating sexual conversion in a subpopulation of parasites. We discovered a single valine (V2163) to leucine (L2163) mutation in an Apetala 2 (AP2) transcription factor required for P. falciparum gametocytogenesis, ap2-g that abrogates sexual differentiation and confirmed this with forward and reverse mutation editing. Mutated AP2-G.L2163 does not bind the ap2-g consensus motif, GnGTAC, or stimulate AP2-G-dependent gene transcription including autoregulation. We then used AP2-G.L2163 parasite lines as tools to demonstrate the critical role of GDV1 in the initial activation of the silent ap2-g locus during the trophozoite to schizont transition in the absence of functional AP2-G and its autoregulation. Additionally, we show that AP2-G.V is required for MSRP1 expression, which can be used to distinguish early and late sexually committed schizonts. Together this work demonstrates that valine2163 in AP2-G plays a critical role in DNA binding, highlighting the functional importance of this specific region for malaria transmission as well as the critical role of GDV1 in the initial activation of ap2-g expression and induction of sexual differentiation. The reporter lines generated allow further study of signaling pathways or screening of factors regulating sexual commitment.