Epstein-Barr virus and Helicobacter pylori Coinfection Drives Gut and Brain Barrier Dysfunction
Epstein-Barr virus and Helicobacter pylori Coinfection Drives Gut and Brain Barrier Dysfunction
Jha, H. C.; Saini, V.; Singh, S.; Baral, B.; Sinha, P.; Singh, A.; Gaudin, R.; Parmar, H. S.
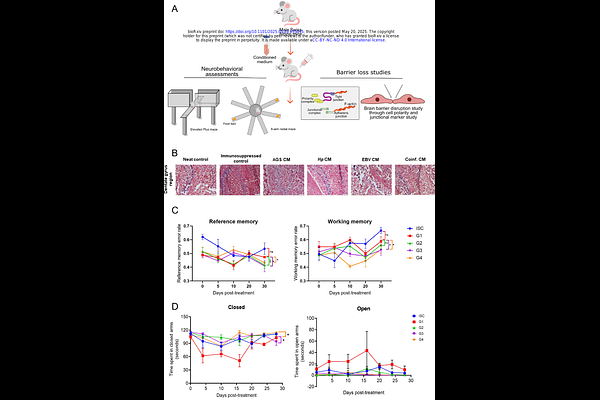
AbstractBiological barriers, including blood-brain barrier (BBB) and gut epithelial barrier, are essential to maintain homeostasis and provide protection against pathogenic stress. Here, we investigated the impact of Helicobacter pylori-Epstein Barr Virus coinfection on gut-brain axis integrity. Our results demonstrate that coinfection induces significant disruptions in tight and adherens junction (TJ and AJ respectively) proteins in gastric epithelial cells, including downregulation of ZO-1 and E-cadherin. The secretome from these coinfected cells significantly compromises the integrity of BBB-derived endothelial cells, including cleavage of VE-cadherin, loss of TJ proteins such as Claudin-5, and enhanced permeability. Furthermore, the sole exposure of mice to coinfected secretome lead to the loss of cerebral VE-cadherin and ZO-1 and the induction of neuroinflammation, characterized by elevated pro-inflammatory cytokines, TNF-, amyloidogenic accumulation, and neurocognitive deficits. The secretome driving such forces potentially carries pathogenic metabolites, host inflammatory cytokines and metabolites that might contribute to disease pathogenesis as observed in this study. Together, our current study provides novel insights into how coinfections contribute to gut-brain axis dysregulation and neurological pathogenesis.