The NOX2-ROS-NLRP3 inflammasome axis in traumatic brain injury
The NOX2-ROS-NLRP3 inflammasome axis in traumatic brain injury
Laabei, J.; Vegliante, G.; Strogulski, N. R.; Douglas, C.; Threja, S.; Pearson, A.; Nkiliza, A.; Hanscom, M.; Filogonio Emediato, I. D.; Crawford, F.; Ojo, J.; Loane, D.
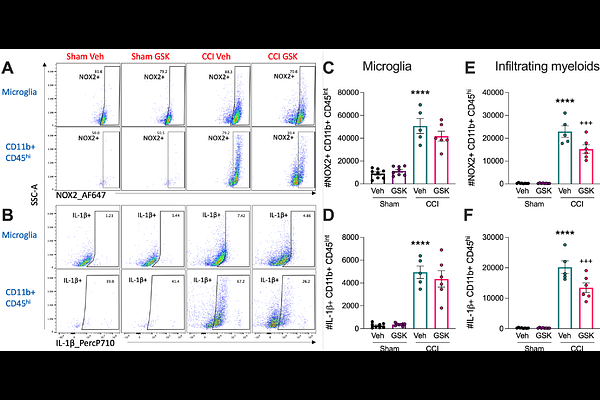
AbstractBackground: Phagocyte NADPH oxidase 2 (NOX2) is an enzyme complex responsible for reactive oxygen species (ROS) production. Chronic NOX2 activity sustains oxidative stress/damage and drives neuroinflammation following traumatic brain injury (TBI). NOX2 acts as a priming signal for NLRP3 inflammasome activation, which also plays a role in secondary injury after TBI. GSK2795039 is a small molecule brain penetrable drug that inhibits NOX2 in a NADPH competitive manner. Here, we investigated whether pharmacological inhibition of NOX2 using GSK2795039 can reduce secondary neuroinflammation after TBI, specifically via inhibition of downstream NLRP3 inflammasome activation, in both resident microglia and infiltrating myeloid cells in the injured brain. Methods: Immortalised microglial (IMG) cells or primary microglia were pre-treated with GSK2795039 (NOX2 inhibitor) or MCC950 (NLRP3 inhibitor) and stimulated with lipopolysaccharide and nigericin to induce NOX2/ROS and NLRP3 inflammasome activation. The controlled cortical impact model, pharmacokinetic analyses, multi-dimensional flow cytometry, histology and neurobehavioral assessments were used to translate in vitro findings to an experimental TBI model in adult male C57BL6/J mice. Results: The small molecule NOX2 inhibitor, GSK2795039, attenuated microglial NOX2 activity, thereby reducing ROS, nitrite and cytokine levels, as well as NLRP3 inflammasome components in pro-inflammatory microglia. TBI recruited NOX2/ROS/IL-1{beta}+ neutrophils and inflammatory monocytes into injured brain parenchyma with peak monocytic NOX2/ROS/IL-1{beta} production at 3 days post-injury (DPI), coincident with peak NOX2/ROS/IL-1{beta} expression in microglia. Systemic administration of GSK2795039 (100mg/kg; I.P.) starting 2 hours post-injury attenuated NOX2/IL-1{beta}+ microglial and infiltrating myeloid cell activation at 3 DPI. In addition, NOX2 inhibition reduced numbers of IL-1R+ T cells in the brain of TBI mice, indicating that myeloid-T cell crosstalk was altered by GSK2795039 treatment. Innate and adaptive neuroimmune changes were associated with improvements in motor function post-TBI. In the chronic phase through 28 DPI, pharmacological inhibition of NOX2 by GSK2795039 treatment resulted in modest improvements in neurobehavioral deficits and TBI neuropathology. Conclusions: These preclinical studies identify the NOX2-ROS-NLRP3 inflammasome axis along with myeloid-T cell crosstalk as effective targets for TBI neuroinflammation. Our translational studies indicate that NOX2 may be a promising therapeutic target for mitigating neuroinflammation in microglia, and peripheral immune cells, following experimental TBI in mice.