Structural Dissection of Vaccinia G9 Identifies Residues Essential for Membrane Fusion and Complex Assembly
Structural Dissection of Vaccinia G9 Identifies Residues Essential for Membrane Fusion and Complex Assembly
Chiu, H.-J.; Chang, W.; Wang, H.-C.
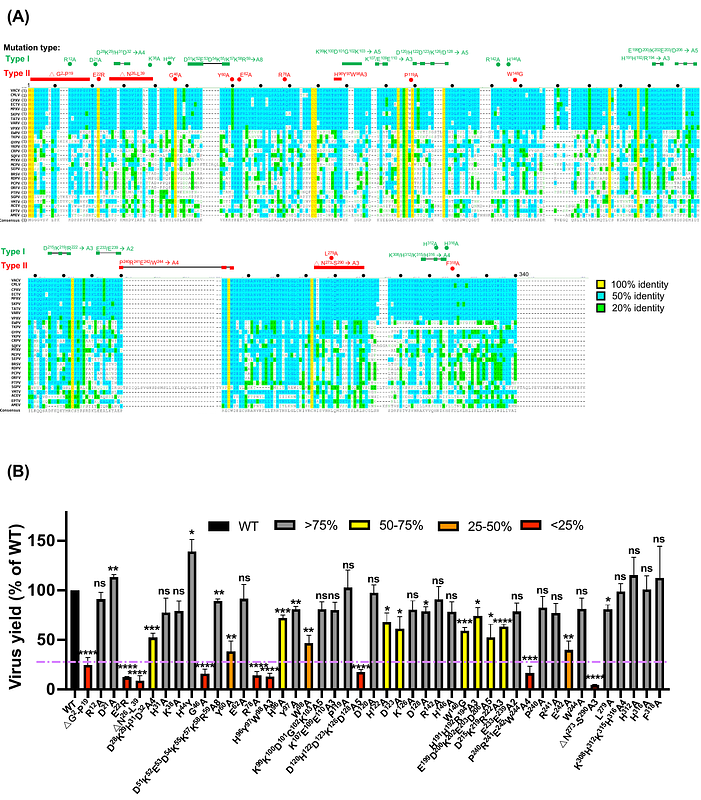
AbstractVaccinia virus, a prototypical poxvirus, utilizes a unique multi-protein Entry Fusion Complex (EFC), comprising 11 components, to mediate membrane fusion during host cell entry. Although the crystal structure of a truncated form of the G9 protein has been determined, the functional relevance of its structural features remains poorly understood. In this study, we systematically analyzed 47 G9 mutants to identify critical functional residues. Using trans-complementation assays, co-immunoprecipitation, membrane fusion assays, and structural analysis, we identified nine key mutants, which were categorized into three functional groups. Group 1 mutants failed to interact with A16 and other EFC components, highlighting their essential roles in G9-A16 subcomplex formation. Group 2 and Group 3 mutants retained A16 binding but disrupted interactions with other EFC proteins, suggesting their roles in broader complex assembly. Notably, Group 3 mutants targeted a conserved P(R/Y)XCW motif and a loop structure shared among vaccinia G9, A16, and J5 proteins. A similar motif was also identified in G9 homologs from Nucleocytoviricota, suggesting an evolutionarily conserved fusion mechanism. Collectively, our findings demonstrated that G9 function requires multiple domains, including A16-binding interfaces and conserved motifs not resolved in previous protein structure. These results establish G9 as a central EFC component and underscore its potential as a target for antiviral development.