The interplay of membrane tension and FtsZ filament condensation on the initiation and progression of cell division in B. subtilis
The interplay of membrane tension and FtsZ filament condensation on the initiation and progression of cell division in B. subtilis
Ramirez-Diaz, D. A.; Yin, L.; Albanesi, D.; Zheng, J.; De Mendoza, D.; Garner, E.
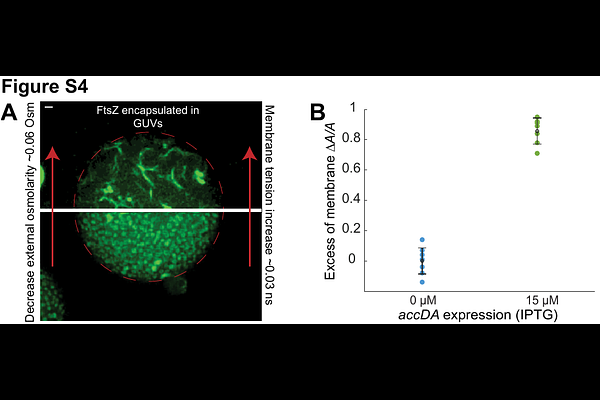
AbstractThe first step of cell division is deforming the planar cell membrane inward towards the cytoplasm. As deforming membranes is energetically costly, biology has developed various protein systems to accomplish this task. The mechanisms providing the force to deform bacterial membranes to initiate division remain unknown. In vivo studies have shown the condensation of FtsZ filaments into a sharp ring is required to initiate cell division, an observation mirrored in vitro with FtsZ filaments encapsulated inside liposomes. Similarly, the force for membrane deformation in many eukaryotic deforming systems arises from the local crowding of proteins on the membrane surface. As any membrane deforming system works against the membrane tension, here we modulated the amount of lipid synthesis and thus membrane tension in Bacillus subtilis to examine: 1) if the condensation of FtsZ filaments by FtsZ bundling proteins serves to overcome the cellular membrane tension to deform the membrane inward and 2) how changes to the membrane tension affect the subsequent invagination of the septum. First, we developed methods to simultaneously measure and modulate membrane tension in live cells. Next, we determined how altering the membrane tension affected the cells ability to initiate division with reduced levels of FtsZ bundling proteins. While cells depleted of 2 FtsZ bundling proteins were unable to divide, reducing membrane tension to a given threshold restored their ability to initiate division. Likewise, cells with intermediate levels of FtsZ bundling proteins required a lesser decrease in membrane tension to initiate division. We also found that reductions in membrane tension increase the rate of Z ring constriction, with the constriction rate scaling linearly with the membrane tension. Interestingly, while the constriction rate in wild-type B. subtilis is limited by FtsZ treadmilling, the rate of constriction becomes independent of FtsZs treadmilling rate when membrane tension is reduced. These experiments give two major insights: First, the filament condensation caused by FtsZ bundling proteins works to overcome membrane tension and deform the membrane inward to initiate division. Second, the rate of septal constriction is limited by membrane tension, suggesting that membrane fluctuations at the tip of the growing septa limit the rate of cell wall synthesis. Finally, our measurements allow the estimation of several physical values of cell division, such as the force required to bend the membrane, but also that the cell membrane provides only 0.1%, a small amount of surface tension relative to the entire cell envelope, indicating 99.9% of the pressure drop occurs across the cell wall. These calculations also indicate that cell division occurs via comparatively very small membrane tension fluctuations relative to the high turgor pressure that exists across the entire cell envelope.