Discovery of Light-Powered Organic Ion Transport by a Natural Protein
Discovery of Light-Powered Organic Ion Transport by a Natural Protein
Shen, S.; Akita, S.; Wada, J.; Eguchi, M.; Tsukamoto, T.; Jung, K.-H.; Sudo, Y.; Kikukawa, T.
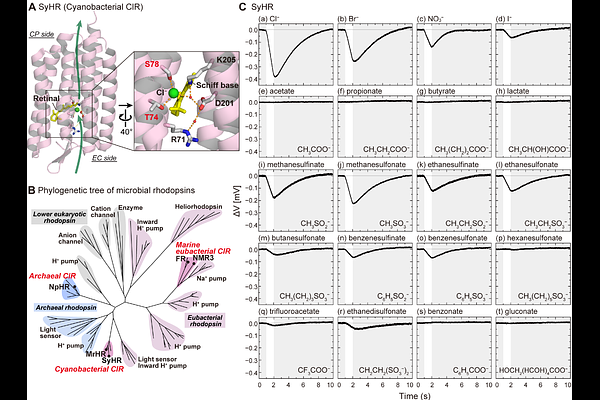
AbstractMembrane transport proteins play vital roles in living cells by selectively transporting ions and molecules across biological membranes. Among these, microbial rhodopsins are unique in their unparalleled capacity to harness light to drive ion translocation. This distinctive feature has led to their widespread use as optogenetic tools in neuroscience, physiology, and biomedical applications. While not all microbial rhodopsins function as ion transporters, many ion-translocating variants have been discovered since the identification of the first member - a light-driven H+ pump - in the 1970s. These proteins share a compact structure composed of only seven transmembrane helices and have long been thought to specialize exclusively in transporting small inorganic ions such as H+, Cl-, and Na+. Here, we show that several anion-pumping microbial rhodopsins can also transport organic anions. In particular, a rhodopsin from cyanobacteria is capable of transporting bulky organic anions, including those containing benzene rings, with molecular volumes up to ~120 [A]3 - five times that of Cl-. These organic ions bind to the dark state and are translocated upon photoactivation, following a mechanism similar to that of inorganic anion transport. Mutational analysis indicates that both classes of substrates share a common binding site. Only anions with pKa values below 2 were transported, suggesting that a retained negative charge is essential for binding to the dark state - a prerequisite for transport. This study expands the known substrate repertoire of microbial rhodopsins and introduces new possibilities for optogenetic strategies based on light-driven delivery of bioactive organic molecules.