Agent-based computational modeling of the stochastic dynamic behavior of actin filaments recapitulates the homeostatic cortical array in plant epidermal cells
Agent-based computational modeling of the stochastic dynamic behavior of actin filaments recapitulates the homeostatic cortical array in plant epidermal cells
Kim, J. H.; Zhang, W.; Iyer-Pascuzzi, A. S.; Staiger, C. J.; Kim, T.
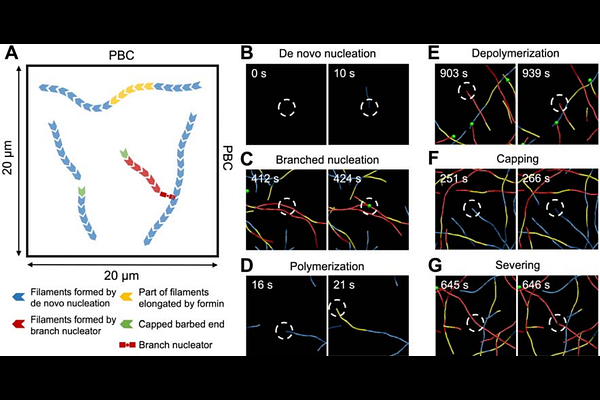
AbstractThe homeostatic cortical actin array in plant cells plays important roles in fundamental processes, including intracellular transport, secretion, cell expansion, and cytoplasmic streaming. In response to diverse chemical and mechanical signals, the cortical array can remodel within minutes to assume new configurations or altered filament abundance. The homeostatic cortical actin array of some plant epidermal cells comprise sparsely distributed individual actin filaments and actin bundles, which enables tracking and quantitative analysis of dynamic properties over many minutes at high spatiotemporal resolution. Previous studies using quantitative live-cell imaging, small molecule inhibitors, and genetic mutations reveal the robust dynamic steady state of the cortical actin array, with individual filaments showing a behavior termed stochastic dynamics. Compared to experimental findings, computational efforts focused on the plant actin cytoskeleton are lacking, although computational models have the potential to define underlying mechanisms of actin array homeostasis and remodeling. Here, we used an agent-based computational model to reproduce the stochastic dynamic behavior of individual actin filaments in epidermal cells with consideration of key governing factors, including the nucleation, polymerization, depolymerization, severing, capping, and branching of filaments. Our model was able to reproduce experimental observations with respect to the abundance and length of filaments as well as the rates or frequencies of dynamic events. This model can be used to study the role of myosin motors and other actin-binding proteins, as well as the effects of signaling events and fluxes in cellular second messengers, on actin dynamics in plant cells.