Spatial activation of Kinesin-1 by Ensconsin shapes microtubule networks via ncMTOCs recruitment
Spatial activation of Kinesin-1 by Ensconsin shapes microtubule networks via ncMTOCs recruitment
Berisha, A.-M.; Pascal, A.; Guelle, M.; clement, B.; Chretien, D.; Bataille, L.; GIET, R.
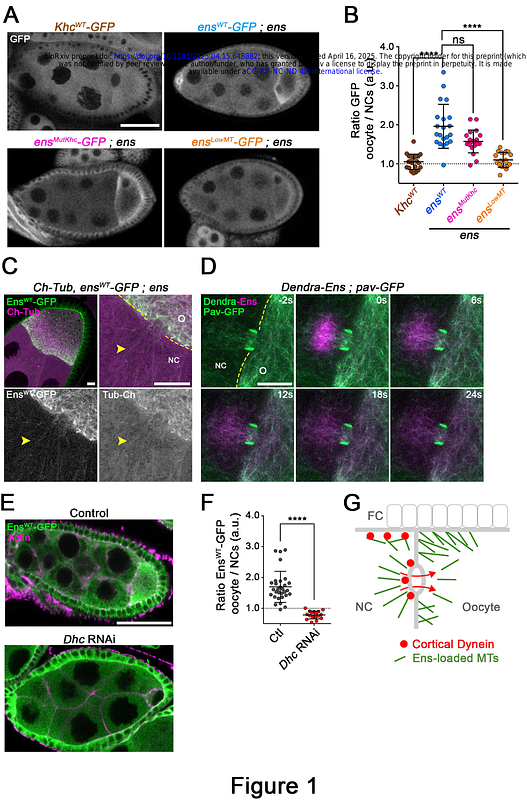
AbstractIntracellular transport, cell morphogenesis, motility, and division require the precise control of the microtubule (MT) cytoskeleton, which varies in shape and dynamic. Drosophila oogenesis requires the formation of cortical growing MTs that produces MTs twisters, sustaining cytoplasmic advection and polarization. Although Kinesin-1 MT motor is a central player regulating these processes, its exact function in the formation of these MTs is elusive. Here, we show that Ensconsin/MAP7, a Kinesin-1 activator, is transported by Dynein together with MTs and maintained at the oocyte cortex by Ninein to regulate spatial activation of Kinesin-1 in the oocyte. Perturbation of this process leads to a severe reduction of ncMTOCs (non-centrosomal MicroTubule Organizing Centers) targeting to the cell cortex, and reduced MTs anchoring and stabilization. We also show that an active Khc variant, unable to bind Ens/MAP7 and harboring a loss of auto-inhibition, is sufficient to restore ncMTOCs recruitment, MTs twister formation and advection in the oocyte. Our findings reveal a pivotal mechanism by which the targeted localization of activators drives the spatial activation of motors, a process fundamental to microtubule remodeling.