Structural and Functional Mechanisms Underlying Activation Gate Dynamics and IFM Motif Accessibility in Human Nav1.5
Structural and Functional Mechanisms Underlying Activation Gate Dynamics and IFM Motif Accessibility in Human Nav1.5
Biswas, R.; Lopez-Serrano, A. L.; Purohit, A.; Ramirez-Navarro, A.; Huang, H.-L.; Cheng, X.; Heissler, S. M.; Deschenes, I.; Chinthalapudi, K.
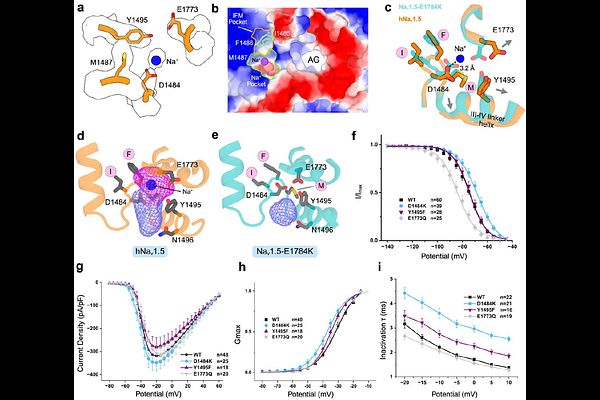
AbstractVoltage-gated sodium channels are vital for regulating excitability in muscle and nerve cells, and their dysregulation is linked to a range of diseases. However, therapeutic targeting of Nav channels remains challenging due to a limited understanding of their gating mechanisms. Here, we present a cryo-EM structure of human Nav1.5 in an intermediate open state, stabilized by interactions between the N-terminal domain and the S6I segment. This structure reveals a previously uncharacterized Na+ binding site adjacent to the conserved inactivation (IFM) motif. Molecular dynamics simulations demonstrate that monovalent cations stably occupy this site, while electrophysiological recordings demonstrate that ion binding modulates IFM motif docking and fast inactivation kinetics. Our findings reveal that IFM accessibility is dynamically regulated in this intermediate state, challenging the canonical hinged-lid model of fast inactivation. Collectively, our study provides a revised structural framework for Nav1.5 gating mechanisms, suggesting an alternative pathway for ion accessibility that may inform better mechanistic and therapeutic strategies for treating Nav1.5-related cardiac arrhythmias.