High-resolution cryo-EM structures of small protein-ligand complexes near the theoretical size limit
High-resolution cryo-EM structures of small protein-ligand complexes near the theoretical size limit
Park, K.; Yoo, Y.; Jeon, H.; Choi, K.; Kwon, E.; Lim, H.-H.; Kim, D. Y.; No, K. T.
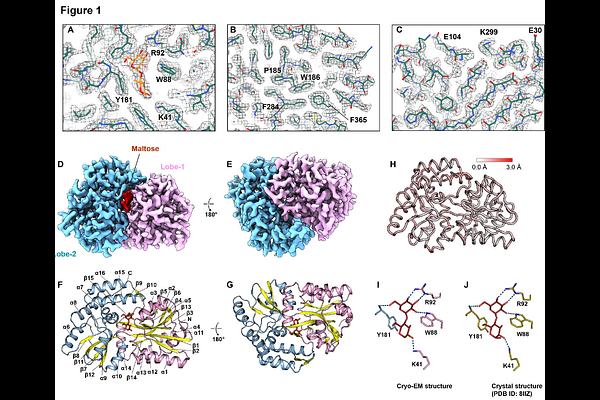
AbstractCryo-electron microscopy (cryo-EM) is widely used to determine macromolecular structures at atomic resolution. The theoretical size lower limit of particles for cryo-EM analysis is 38 kDa, limited by factors such as contrast and particle alignment accuracy. To date, no cryo-EM structures have been reported for proteins near this size limit. This study presents cryo-EM structures of two protein-ligand complexes around 40 kDa. The structure of maltose-binding protein (43 kDa) was determined at 2.32 [A] resolution, clearly revealing the bound maltose and water molecules. Additionally, the kinase domain of human PLK1 (37 kDa), slightly below the theoretical limit, was determined at 3.04 [A] resolution, allowing the identification of the bound ligand, onvansertib. These findings demonstrate that cryo-EM can be effectively employed for structure determination and structure-based drug screening of small proteins or domains.