Disruption of spindle orientation and protein localization during asymmetric cleavage by pharmacological inhibition of serotonin signaling
Disruption of spindle orientation and protein localization during asymmetric cleavage by pharmacological inhibition of serotonin signaling
Nakamoto, A.; Nagy, L.
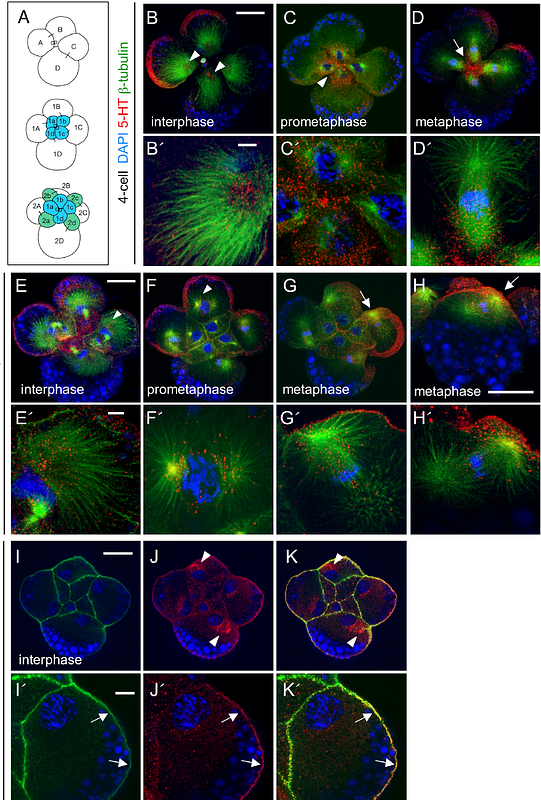
AbstractCell polarity directs the orientation and size of asymmetric cell divisions, and the segregation of cell fate determinants processes fundamental to development in all multicellular organisms. During asymmetric cleavage, the mitotic spindle aligns with a specified polarity of the mother cell, and cell fate determinants are distributed asymmetrically along the division axis. Here, we report that pharmacological inhibition of serotonin signaling during the 4-to-8-cell division in early embryos of the mud snail Ilyanassa obsoleta (currently known as Tritia obsoleta) disrupts the typical unequal division pattern. The oblique axis of division common to spirally cleaving molluscan embryos is altered, and the position of the mitotic spindle is randomized in these treatments. Mother cells generate abnormally large, atypically positioned daughter cells. We also find that, in normal embryos, proteins recognized by phosphorylated PKC and Bazooka/PAR-3 antibodies typically co-localize with the spindle apparatus to the apical cortex of each mother cell. These antigens subsequently segregate to the smaller of the two daughter cells. In embryos treated with the serotonin-receptor antagonist, the localization of these asymmetrically segregating proteins is randomized, and their localization is independent of spindle position. These results suggest that serotonin signaling coordinates spindle orientation, cortical polarity, and cell size in early asymmetric cleavages.