Secretory carrier membrane proteins assist with aquaporin trafficking in Arabidopsis.
Secretory carrier membrane proteins assist with aquaporin trafficking in Arabidopsis.
Jiang, Q.; Vandorpe, M.; fox, a. R.; Vermeersch, M.; Mylle, E.; Cuadrado, A. F.; Kraus, J.; Liu, H.; Eeckhout, D.; Navarre, C.; Courtoy, A.; Jacobs, T. B.; Dragwidge, J. M.; De Smet, I.; Pleskot, R.; Chaumont, F.; Van Damme, D.
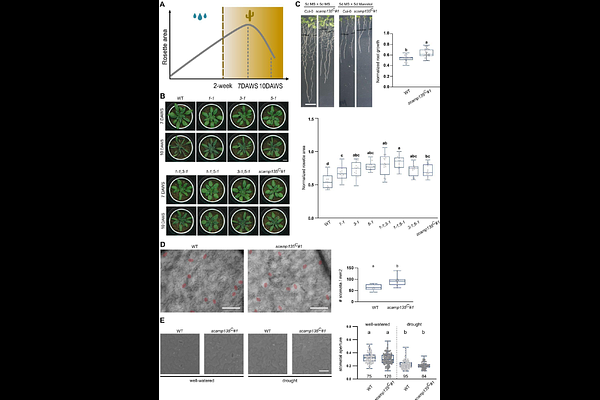
AbstractSecretory carrier membrane proteins are evolutionarily conserved multi-spanning transmembrane proteins. In animal cells, they function in secretion, endocytosis and autophagy. Knowledge on their role in plants is restricted to heterologous localization and trafficking experiments as well as to genetic perturbation experiments in lily and poplar. Here, we analysed the five members of the Arabidopsis SCAMP family. Using SCAMP5, the most prominently expressed member of the family as hallmark, we identified three conserved tyrosine motifs that assist in anterograde transport from the Trans Golgi Network to the plasma membrane, while N-terminally located NPF motifs are crucial for endocytosis. SCAMPs dimerize both at the plasma membrane and endosomes, and dimerization is required for internalization. Functionally, SCAMP5 interacts with several plasma membrane associated aquaporins (PIPs), and is required for their intracellular trafficking as mCherry-PIP2;1 accumulated differentially and specifically in the root meristematic vacuoles independent of the endocytic or secretory trafficking capacity of SCAMP5. Triple and quintuple scamp mutants show only mild developmental delay under standard growth conditions, but are more tolerant to drought. In conclusion, our research links SCAMP proteins to PIPs and we propose that the drought tolerance phenotype of the scamp135 mutants is at least partially caused by altered PIP trafficking.