The bacterial ESCRT-III PspA rods thin lipid tubules and and increase membrane curvature through helix α0 interactions
The bacterial ESCRT-III PspA rods thin lipid tubules and and increase membrane curvature through helix α0 interactions
Hudina, E.; Schott-Verdugo, S.; Junglas, B.; Kutzner, M.; Ritter, I.; Hellmann, N.; Schneider, D.; Gohlke, H.; Sachse, C.
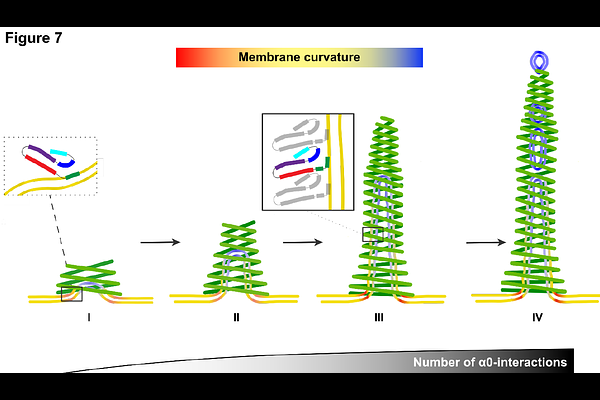
AbstractThe phage shock protein A (PspA), a bacterial member of the ESCRT-III superfamily, forms rod-shaped helical assemblies that internalize membrane tubules. The N-terminal helix 0 of PspA (and other ESCRT-III members) has been suggested to act as a membrane anchor, the detailed mechanism, however, of how it binds to membranes and eventually triggers membrane fusion and/or fission events remains unclear. By solving a total of 15 cryo-electron microscopy (cryo-EM) structures of PspA and a truncation lacking the N-terminal helix 0 in the presence of EPL membranes, we show in molecular detail how PspA interacts with and remodels membranes: binding of the N-terminal helix 0 in the outer tubular membrane leaflet induces membrane curvature, supporting membrane tubulation by PspA. Detailed molecular dynamics simulations and free energy computations of interactions between the helix 0 and negatively charged membranes suggest a compensating mechanism between helix/membrane interactions and the energy contributions required for membrane bending. The energetic considerations are in line with the membrane structures observed in the cryo-EM images of tubulated membrane vesicles, fragmented vesicles inside tapered PspA rods, and shedded vesicles emerging at the thinner PspA rod ends. Our results provide insights into the molecular determinants and a potential mechanism of vesicular membrane remodeling mediated by a member of the ESCRT-III superfamily.