Spatially Tuned Localization of Interleukins and OX40 Agonists EnhancesSynergistic Anti-Tumor Immunity
Spatially Tuned Localization of Interleukins and OX40 Agonists EnhancesSynergistic Anti-Tumor Immunity
Klich, J.; Nejatfard, A.; Meany, E.; Ou, B. S.; Baillet, J.; Appel, E.
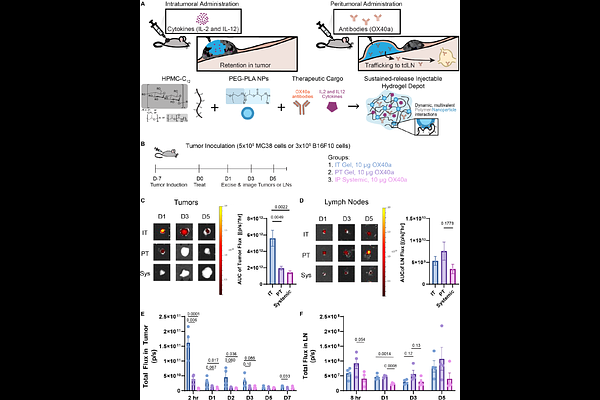
AbstractAdvances in immunotherapy have revolutionized the current standard of care for cancer patients, but unfortunately, most approaches still fail to mount a robust anti-cancer effect. This is in part due to a highly immunosuppressive tumor microenvironment which has developed bio-orthogonal mechanisms of immune escape. To address this challenge, the field has turned to combination immunotherapies, but systemic administration of potent combination therapies has resulted in severe immune related adverse effects and toxicity. Enabling potent combination immunotherapies requires administering these immune agonists in a way that more closely resembles the endogenous cancer immunity cycle, a tightly regulated sequence of cues in both space and time. Here, we explore the ability of an injectable hydrogel depot to enable the rational localization of potent immunotherapeutic cytokines (IL-12, IL-2) and antibodies (OX40a). We hypothesized that selectively altering the biodistribution of these cargo would enable tolerable and synergic anticancer combinations, so we leveraged a previously characterized injectable polymer-nanoparticle (PNP) hydrogel system to deliver these agonists either intratumorally (IT) or peritumorally (PT). Using in vivo imaging, we demonstrated that site of administration is critical to redistributing cargo to either the tumor or tumor draining lymph node (tdLN). Further, we demonstrated that the targeted localization of cytokine and antibody therapies synergistically improved treatment efficacy in the B16F10 and MC38 tumor models and altered cellular phenotypes in these microenvironments. This approach thus represents a crucial new strategy for basic cancer immunology and materials-based immuno-engineering research while improving therapeutic efficacy.