SpoIIE drives asymmetric cell division in B. subtilis by sequential modulation of the cytokinesis machinery
SpoIIE drives asymmetric cell division in B. subtilis by sequential modulation of the cytokinesis machinery
Ryan, A.; Squyres, G. R.; Holmes, M. J.; Bisson, A.; Garner, E. C.; Bradshaw, N.
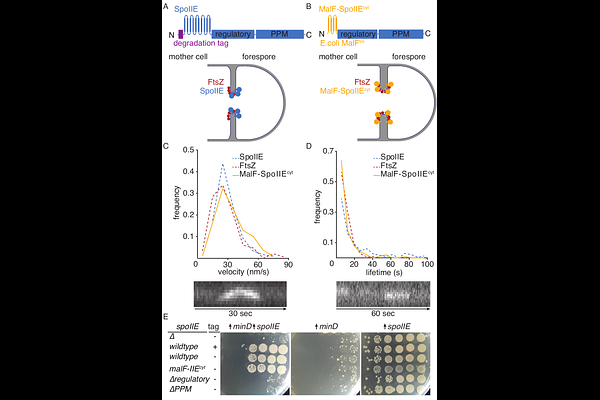
AbstractTo form a dormant spore, Bacillus subtilis and related endospore-forming bacteria divide asymmetrically to generate daughter cells of unequal size, the smaller of which becomes the spore. The transmembrane protein SpoIIE repositions the cell division machinery and controls cytokinesis during sporulation, but the molecular basis for the precise placement of the asymmetrical division site to the quarter cell point is unknown. Here, we applied live-cell fluorescence microscopy techniques to reveal that SpoIIE localizes with the treadmilling components of the cell division machinery. We found that SpoIIE opposes the inhibitory activity of the MinCD complex, which prevents assembly of Z-rings near the cell poles. Cells expressing a variant of SpoIIE with its transmembrane region replaced by an unrelated transmembrane anchor assembled condensed Z-rings that were unable to initiate constriction. This reveals a new function of SpoIIE and a possible checkpoint licensing cytokinesis downstream of Z-ring condensation. Potentially explaining the role of SpoIIE in cytokinesis, we demonstrated that SpoIIE\'s transmembrane region interacts with DivIB, an enigmatic structural component of the cell wall synthesis complex required for cytokinesis during sporulation. Finally, we found that FtsZ filaments are unusually short during sporulation, which requires the transmembrane domain of SpoIIE. Together, these results demonstrate that SpoIIE sequentially influences polar divisome assembly at distinct steps to drive asymmetric cell division.