Kmo restricts Salmonella in a whole organism infection model by promoting macrophage lysosomal acidification through kainate receptor antagonism.
Kmo restricts Salmonella in a whole organism infection model by promoting macrophage lysosomal acidification through kainate receptor antagonism.
Goering, E. R.; Clatworthy, A. E.; Parada-Kusz, M.; Bagnall, J.; Hung, D. T.
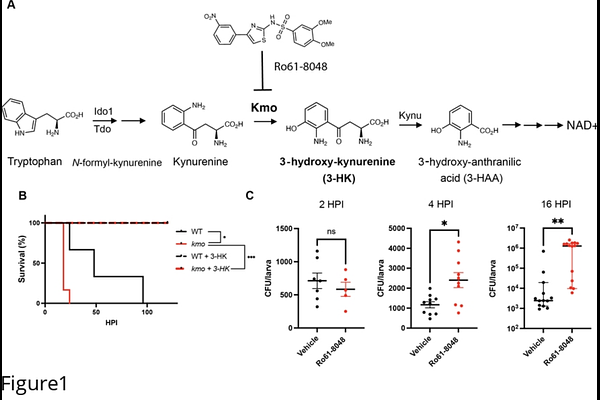
AbstractThe kynurenine pathway of tryptophan degradation has been implicated in various diseases, including cancer, neurodegenerative disorders, and infectious diseases. A key branchpoint in this pathway is production of the metabolite 3-hydroxy-kynurenine (3-HK) by the enzyme kynurenine 3-monooxygenase (Kmo). We have previously reported that administration of exogenous 3-HK promotes survival of zebrafish larvae to Salmonella Typhimurium infection by restricting bacterial expansion via a systemic mechanism that targets kainate sensitive glutamate receptor (KAR) ion channels and that the endogenous production of 3-HK by Kmo is required for defense against systemic Salmonella infection. Here we show that endogenous 3-HK promotes lysosomal acidification to contribute to macrophage microbicidal activity, with its absence leading to increased host susceptibility to infection. Further, 3-HK promotes lysosomal acidification in a KAR-dependent manner. We thus reveal a novel link between KARs and macrophage lysosomal acidification, and a novel mechanism by which 3-HK promotes control of bacterial infection.