The accessory protein CvnF8 modulates histidine kinase activity in an Actinobacterial G protein system in Streptomyces coelicolor
The accessory protein CvnF8 modulates histidine kinase activity in an Actinobacterial G protein system in Streptomyces coelicolor
Cantu Morin, L. M.; Dekoninck, K.; Min, K.-Y.; Traxler, M. F.
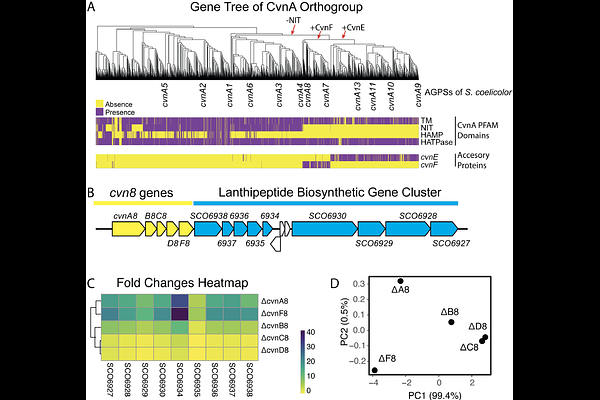
AbstractConservons are regulatory systems found in bacteria of the phylum Actinomycetota. These regulatory systems are composed of four core proteins: a sensor histidine kinase-like protein (CvnA homolog), an MglA-type roadblock protein (CvnB homolog), a protein containing a domain of unknown function (CvnC homolog), and small Ras-like GTPase (CvnD homolog). Based on their conserved small GTPase components and their phylogenetic distribution, we propose that conservons should be known as Actinobacterial Gprotein systems (AGPSs). The signal transduction path through AGPSs remains poorly understood, and some AGPSs have additional accessory proteins (CvnE and CvnF homologs) of unknown function. In this work, we show that AGPS accessory proteins are present when the cognate histidine kinase protein (CvnA homolog) lacks an extracytoplasmic sensory domain. It was previously shown that the Cvn8 AGPS of Streptomyces coelicolor controls expression of multiple pathways for specialized metabolism and that the Cvn8 AGPS contains an accessory protein, CvnF8. Through protein modeling, we found that CvnF8 may share an interaction interface with the histidine kinase CvnA8, prompting the hypothesis that CvnF8 may serve as a modulator of CvnA8 activity. We found that in a purified system, CvnF8 strongly stimulated the ATPase activity and autophosphorylation of CvnA8. Taken together, these findings indicate that CvnF family accessory proteins likely serve as sensors and/or modulators of histidine kinases of AGPSs found broadly in Actinomycetota.