A secreted fatty acid- and retinol- binding protein from Heligmosomoides polygyrus suppresses host macrophage polarization
A secreted fatty acid- and retinol- binding protein from Heligmosomoides polygyrus suppresses host macrophage polarization
Azizpor, P.; Montoya, J.; Eyabi, F.; Ramirez, J.; Hill, T.; Pena, R.; Mishra, M.; Boulanger, M.; Dillman, A. R.
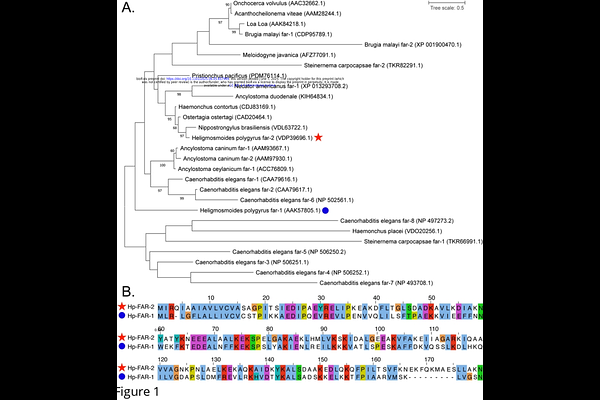
AbstractParasitic nematodes are major pathogens of humans, animals, and plants, contributing to global health challenges and substantial agricultural losses. Fatty acid- and retinol-binding proteins (FARs), secreted by parasitic nematodes, are believed to play key roles in host-pathogen interactions, including immune modulation and nutrient acquisition. In this study, we characterize a FAR protein from the gastrointestinal nematode Heligmosomoides polygyrus, Hp-FAR-2. Unlike FARs from Caenorhabditis elegans, Steinernema carpocapsae, and Ancylostoma ceylanicum, Hp-FAR-2 did not influence immunity or survival in a Drosophila melanogaster infection model, suggesting functional divergence within the FAR family. Competitive lipid-binding assays revealed a preference for omega-3 and omega-6 polyunsaturated fatty acids indicating selective binding to bioactive lipids that may modulate immunity. Using RAW 264.7 macrophages, we found that Hp-FAR-2 suppresses the expression of both M1-associated (TNF-, IL-6) and M2-associated (Chil3) markers during polarization, implicating it as a broad immunomodulator that may inhibit inflammatory responses and tissue repair mechanisms to promote chronic infection. Our findings support a model in which Hp-FAR-2 disrupts host lipid signaling and immune function to favor parasite persistence, suggesting its potential role in the excretory/secretory products of H. polygyrus. Together, these data enhance our understanding of FAR-mediated host manipulation and may inform the development of novel anthelmintic or immunoregulatory therapies.