High-Level Expression, Purification and Biophysical Characterization of GPI-anchored native-like human Prion Protein using Leishmania tarentolae
High-Level Expression, Purification and Biophysical Characterization of GPI-anchored native-like human Prion Protein using Leishmania tarentolae
Bolakhrif, N.; Pauly, T.; Nagel, L.; Willbold, D.; Gremer, L.
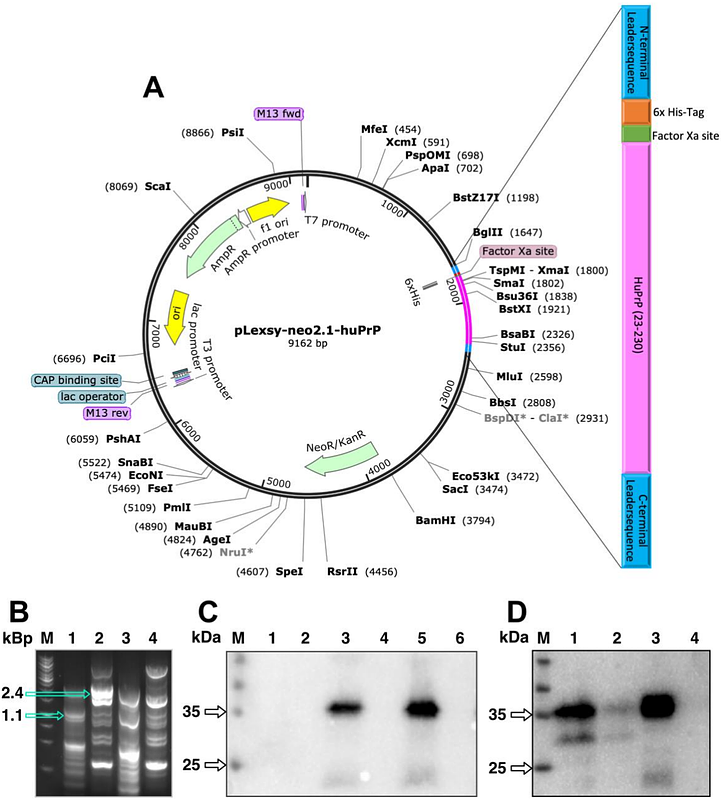
AbstractThe human prion protein (PrP) is a glycosylphosphatidylinositol (GPI)-linked membrane-bound glycoprotein, containing two glycosylation sites. Human PrP is associated with a number of neurodegenerative diseases, called transmissible spongiform encephalopathies (TSE). Pathogenesis involves a structural conversion of the cellular form (PrPC), rich in -helical and random coil structure, into the scrapie form (PrPSc) characterized by parallel in register intermolecular {beta}-sheet conformation. To get a better understanding of this structural conversion, it is crucial to first characterize the non-pathogenic cellular isoform including all posttranslational modifications, like GPI-anchoring and native-like human glycosylation pattern. So far, studies on PrPC or PrPSc as well as the transition from one state to the other rely on non-native constructs of PrP studied far away from physiological conditions. We, therefore, established the expression of GPI-linked human PrP with close to native glycosylation pattern (native-like human PrP) using the eukaryotic LEXSY expression system in Leishmania tarentolae. This expression system has the added advantage that it allows for large-scale production of the native-like human PrP, which results in ~1 mg purified protein per liter culture. Sedimentation velocity analysis and far-UV circular dichroism spectroscopy confirm the high structural homogeneity and monomeric native-like conformation of the purified GPI-anchored human PrP.