A key spectral tuning site of UV-sensitive vertebrate non-visual opsin Opn5
A key spectral tuning site of UV-sensitive vertebrate non-visual opsin Opn5
Yamashita, T.; Asamoto, K.; Fujii, K.; Fujiyabu, C.; Ohuchi, H.; Shichida, Y.
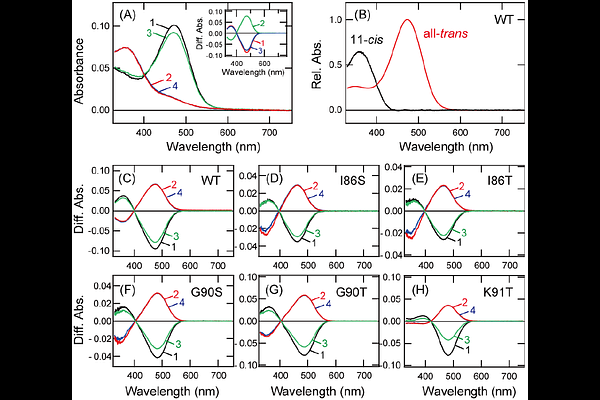
AbstractOpsins are photoreceptive proteins responsible for visual and non-visual photoreceptions in animals. In general, vertebrates have multiple visual and non-visual opsins whose spectral sensitivities range from the UV to the red region. Among these opsins, Opn5 has been widely identified in vertebrates from fishes to primates and functions as a non-visual opsin in various tissues including the retina and brain. Vertebrate Opn5 has been characterized as a UV-sensitive bistable opsin. Thus, Opn5 provides one of the molecular mechanisms determining the short wavelength limit that vertebrates can detect. In this study, we searched for the amino acid residue responsible for the UV light sensitivity of Opn5. Our mutational analysis revealed that Opn5 acquired visible light sensitivity by the substitution of Lys91 with an amino acid other than arginine or tyrosine residue. In addition, the mutations at Lys91 altered the preferential binding of the retinal isomers in Opn5. Therefore, the conservation of Lys91 among vertebrate Opn5 proteins would be necessary to enable Opn5 to work as the shortest wavelength sensor in various tissues.