Chromatin Remodeling and Transcriptional Silencing Define the Dynamic Innate Immune Response of Tissue Resident Macrophages After Burn Injury.
Chromatin Remodeling and Transcriptional Silencing Define the Dynamic Innate Immune Response of Tissue Resident Macrophages After Burn Injury.
Kim, H. G.; Gauthier, M.-P. L.; Higgs, A.; Hernandez, D. A.; Zhou, M.; Brant, J. O.; Bacher, R. L.; Darden, D. B.; Wallet, S. M.; Mathews, C. E.; efron, P. A.; Kladde, M. P.; Maile, R.
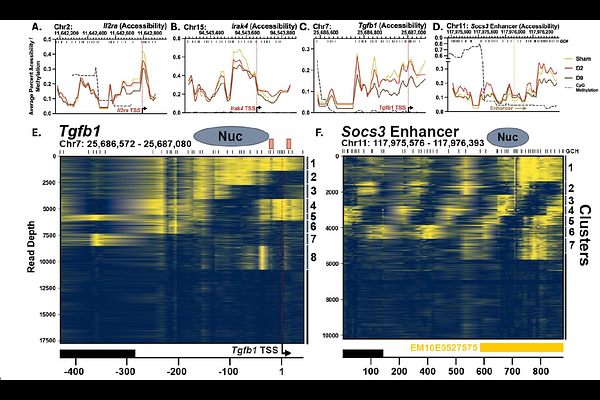
AbstractSevere burn injury induces long-lasting immune dysfunction, but the molecular mechanisms underlying this phenomenon remain unclear. We hypothesized that burn injury leads to epigenetic and transcriptional reprogramming of innate immune cells. Splenic F4/80 macrophages were isolated from mice at days 2, 9, and 14 days post-20% contact burn injury. Targeted transcriptomics and MAPit single-molecule chromatin profiling were used to assess immune, metabolic, and epigenetic changes. Canonical pathway analysis was performed to infer functional shifts over time. Burn injury induced a biphasic response in macrophages. Early after injury (Day 2), there was broad transcriptional suppression and epigenetic silencing of inflammatory regulators, including Stat3, Traf6, and Nfkb1. Over time (Days 9 and 14), loci associated with anti-inflammatory mediators such as Il-10 and Socs3 exhibited progressive chromatin opening and transcriptional upregulation. Metabolic gene profiles revealed persistent suppression of mitochondrial and oxidative phosphorylation programs. Canonical pathway analysis demonstrated early IL-10 signaling activation with sustained suppression of classical macrophage activation pathways. Chromatin architecture changes included nucleosome sliding and ejection events, consistent with dynamic, locus-specific regulation. This work challenges the classical notion of burn-induced immune suppression as purely a consequence of systemic inflammation. Instead, we reveal a programmed and locus-specific epigenetic architecture that may shape macrophage immune and metabolic function long after the acute phase.