A reversible feedback mechanism regulating mitochondrial heme synthesis
A reversible feedback mechanism regulating mitochondrial heme synthesis
Chitrakar, I.; Roberson, A. B.; Ayres-Galhardo, P. H.; Brown, B. L.
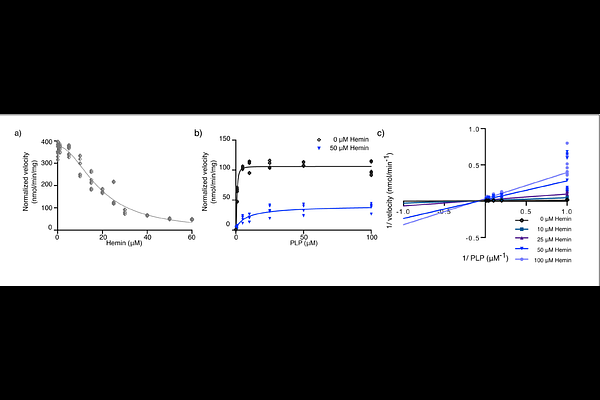
AbstractProper heme biosynthesis is essential for numerous cellular functions across nearly all life forms. In humans, dysregulated heme metabolism is linked to multiple blood diseases, neurodegeneration, cardiovascular disease, and metabolic disorders. Erythroid heme production begins with the rate-limiting enzyme Aminolevulinic Acid Synthase (ALAS2) in the mitochondrion. Although prior studies discuss the regulation of ALAS2 in the nucleus and cytoplasm, its modulation as a mature mitochondrial matrix enzyme remains poorly understood. We report that heme binds mature human ALAS2 with high affinity, acting as a reversible mixed inhibitor that reduces enzymatic activity. Structure-based modeling reveals two flexible regions of ALAS2 interact with heme, locking the enzyme in an inactive conformation and occluding the active site. Our work reveals a negative feedback mechanism for heme synthesis, offering insights into the spatial regulation of ALAS2 and the maturation of the essential heme cofactor.