Identification and Rescue of Congenital Hyperinsulinism-Associated ABCC8 Mutations that Impair KATP Channel Trafficking
Identification and Rescue of Congenital Hyperinsulinism-Associated ABCC8 Mutations that Impair KATP Channel Trafficking
ElSheikh, A.; Kuo, Y.-Y.; E. Boodhansingh, K.; Yang, Z.; A. Stanley, C.; De Leon, D. D.; Shyng, S.-L.
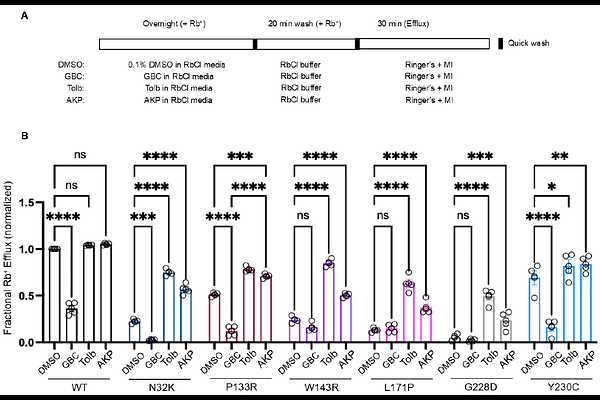
AbstractATP-sensitive potassium (KATP) channels composed of Kir6.2 and sulfonylurea receptor 1 (SUR1) couple glucose metabolism with insulin secretion in pancreatic {beta}-cells and are vital to glucose homeostasis. Loss-of-function mutations in SUR1 and Kir6.2, encoded by ABCC8 and KCNJ11, respectively are the commonest causes of severe persistent hypoglycemia in infants and children seen in the rare disease congenital hyperinsulinism (HI). The N-terminal transmembrane domain, TMD0, and the linker immediately C-terminal to TMD0, L0, of SUR1 (TMD0/L0) forms direct contact with Kir6.2 in KATP channels. Mutations in SUR1-TMD0 often impair KATP channel trafficking to the plasma membrane, causing severe disease unresponsive to treatment by the KATP activator diazoxide; however, surface expression and function of many such mutant channels can be rescued by reversible KATP inhibitor pharmacochaperones. Here, we identified seven new SUR1 missense mutations in TMD0/L0 from HI patients unresponsive to diazoxide and investigated their effects on KATP channel expression, function, and response to pharmacochaperones. All seven mutations, N32K, Y124F, P133R, W143R, L171P, G228D, and Y230C, reduced channel function in Rb+ efflux assays. Further characterization by immunoblotting, immunostaining and electrophysiology revealed that Y124F primarily causes defective channel gating, while the others impair channel trafficking to different extents. The trafficking mutations showed varied response to surface expression and function rescue by the reversible KATP inhibitor pharmacochaperones, tolbutamide and Aekatperone. The study underscores the critical role of SUR1-TMD0/L0 in KATP expression and gating. It further highlights the importance of detailed biochemical and functional studies of mutant channels in understanding their pathogenic roles and response to potential pharmacological therapies.