Placental Iron Utilisation in Fetal Growth Restriction: Alterations in Mitochondrial Heme Synthesis and Iron-Sulfur Cluster Assembly Pathways
Placental Iron Utilisation in Fetal Growth Restriction: Alterations in Mitochondrial Heme Synthesis and Iron-Sulfur Cluster Assembly Pathways
Botha, V. B.; Murray, H. C.; Acharya, S.; Pringle, K. G.; Smith, R.; Fisher, J. J.
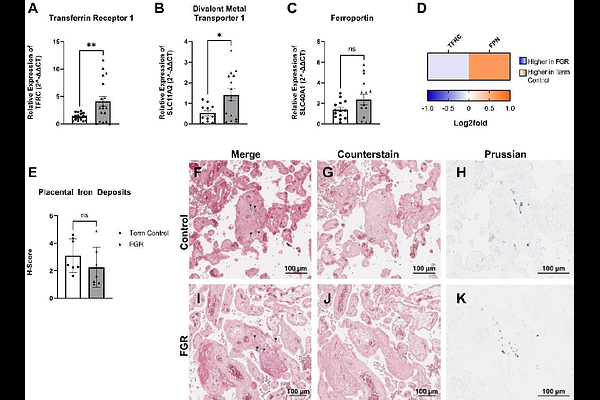
AbstractFetal growth restriction (FGR) affects ~10% of pregnancies worldwide and is often associated with placental insufficiency. Iron is essential for maternal haematopoietic adaptations and placental processes such as mitochondrial iron-sulfur (Fe-S) cluster assembly, heme synthesis, and erythropoiesis. This study aimed to characterise iron transport and downstream utilisation in FGR. Placental tissues from term uncomplicated (n=19) and FGR (n=18) pregnancies were analysed. Maternal iron status was retrospectively assessed from clinical records. Placental mRNA and protein expression of iron-dependent pathways were analysed via RT-qPCR, LC-MS, and Western blotting. Placental iron content was assessed histologically, and heme levels were measured by activity assay. FGR pregnancies showed significantly elevated maternal serum ferritin and lower red cell distribution width, although remained within normal clinical values. Placental iron uptake transporters TFRC and DMT1 were significantly upregulated, while the iron exporter to the fetus, ferroportin, was reduced, indicating increased iron retention in the FGR placenta. Despite altered transporter expression, Fe3 iron levels were unchanged, suggesting iron utilisation over storage. Subsequent investigations identified reduced mitochondrial Fe-S synthesis components (FDXR, FDX2, NDUFAB1, HSPA9), and a prioritisation of mitochondrial and cytosolic heme synthesis enzymes in FGR. Protein levels of haemoglobin subunits (HBG1, HBG2, HBB, HBA1) and erythrocyte membrane markers (EPB41, EPB42, SPTA1, SPTB, ANK1) were decreased. These findings reveal a compensatory response in FGR placentae, with increased iron uptake and utilisation favouring heme synthesis over Fe-S cluster formation, possibly to support oxygen handling under poor placental vascularisation and reduced fetal oxygenation, with potential consequences for mitochondrial energy metabolism.