Graphene Micro-transistor Arrays Reveal Perfusion-Dependent Electrophysiological and Haemodynamic Signatures of Cortical Spreading Depolarizations in Ischaemic Stroke
Graphene Micro-transistor Arrays Reveal Perfusion-Dependent Electrophysiological and Haemodynamic Signatures of Cortical Spreading Depolarizations in Ischaemic Stroke
Flaherty, S. M.; Eladly, A.; Hills, K. E.; Merlini, J. E.; Masvidal-Codina, E.; Fernandez, E.; Illa, X.; Prats-Alfonso, E.; del Corro, E.; Garrido, J. A.; Kostarelos, K.; Allan, S. M.; Guimera-Brunet, A.; Wykes, R.
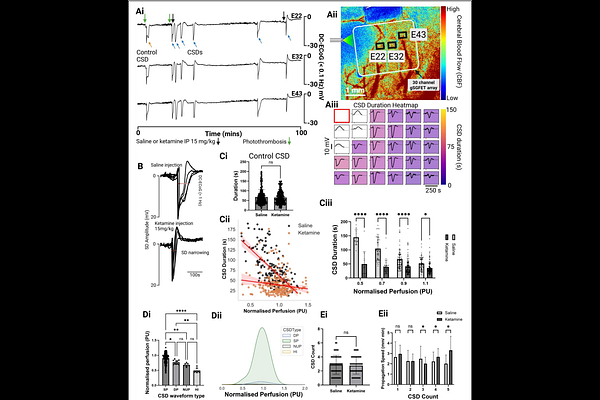
AbstractCortical spreading depolarizations (CSDs) are large scale disruptions of neuronal homeostasis that contribute to secondary injury in ischaemic stroke. In healthy cortex, CSDs induce vasodilation to meet metabolic demand, whereas in ischaemic tissue, they can provoke vasoconstriction and sustained hypoperfusion, exacerbating damage. We present a multimodal neurotechnology platform integrating flexible, transparent graphene solution-gated field-effect transistor (gSGFET) arrays with laser speckle contrast imaging (LSCI), enabling simultaneous DC coupled electrophysiological recordings and cerebral blood flow imaging with high spatiotemporal fidelity. gSGFETs enable stable, distortion-free recording of infraslow potentials essential for resolving CSD waveforms in vivo. In two murine stroke models, we show that CSD waveform duration and morphology scale with local perfusion, delineating electrophysiological subtypes predictive of tissue viability and haemodynamic response. In metabolically compromised cortex, CSDs exhibit double peaks or negative ultraslow components that result in vasoconstriction, contrasting with narrow, monophasic waveforms eliciting vasodilation in healthy regions. Systemic ketamine shortens CSD duration and converts vasoconstriction to vasodilation in perfusion deficit tissue, revealing a mechanism for its neuroprotective action. This platform enables high-fidelity investigation of brain blood flow interactions in vivo.