Fhod3 in zebrafish supports myofibril stability during growth of embryonic skeletal muscle
Fhod3 in zebrafish supports myofibril stability during growth of embryonic skeletal muscle
Russell, A.; Eng, T.; Amack, J. D.; Pruyne, D.
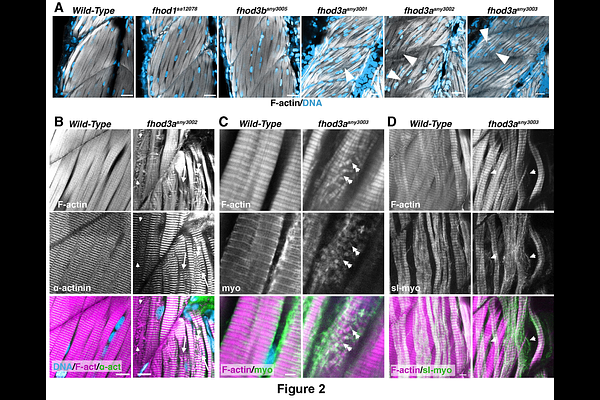
AbstractBackground: Actin filament organization in cardiomyocytes critically depends on the formin Fhod3, but no role has been previously described for Fhod3 in skeletal muscle development. Results: We demonstrate here that in zebrafish mutated for one of two fhod3 paralog genes, fhod3a, skeletal muscle of the trunk appears normal through 2 days post-fertilization, but afterward exhibits myofibril damage, including gaps between myofibrils and myofibril fragmentation. Although myofibril damage appears progressive, fhod3a mutant embryos differ from muscular dystrophy models in that damage is not activity-dependent, but is exacerbated by inhibition of muscle activity. Moreover, fhod3a mutants show no evidence of sarcolemma disruption. Rather, our results suggest myofibril damage coincides with growth of the skeletal muscle fiber contractile apparatus. Neither the second paralog, fhod3b, nor the more distantly related fhod1 appear to contribute to embryonic skeletal muscle development, but simultaneous mutation of fhod3a and fhod3b was associated with pericardial edema suggestive of cardiac dysfunction. Conclusions: Taken together, these results indicate a fhod3 homolog is dispensable for initial myofibril assembly in skeletal muscle, but promotes myofibril stability during muscle fiber growth. This is the first demonstration that Fhod3 contributes to skeletal muscle development in a vertebrate.