Amniotic fluid extracellular vesicle properties evolve with gestational age and reflect fetal development
Amniotic fluid extracellular vesicle properties evolve with gestational age and reflect fetal development
Atukorala, I.; Beard, S.; Ang, C.-S.; Brown, H. G.; Raghavan, S.; de Alwis, N.; Fato, B.; Binder, N.; Hannan, N.; Hui, L.
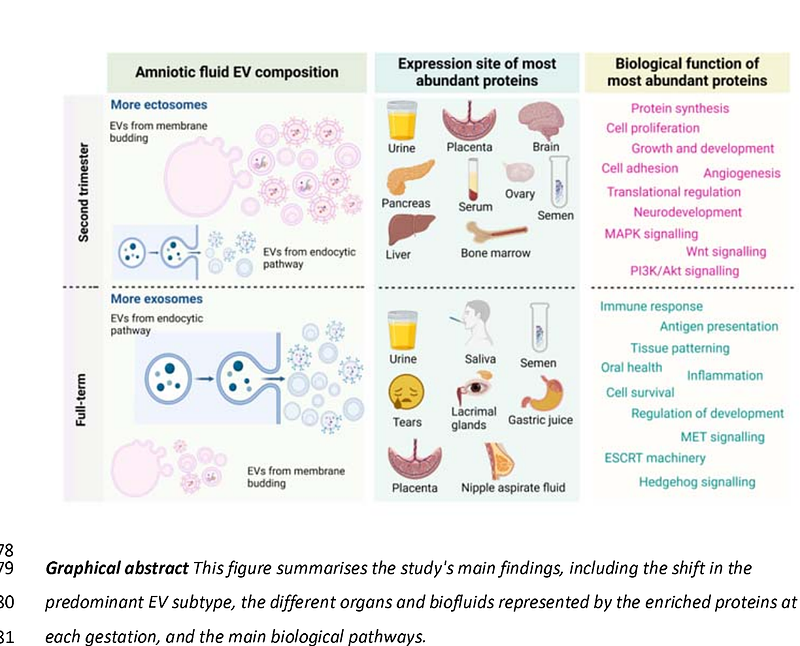
AbstractAmniotic fluid (AF) is a valuable source of extracellular vesicles (EVs) derived from the fetoplacental unit. Preclinical and clinical studies have highlighted promising applications of AF-EVs and their role in cellular communication, yet our understanding of AF-EV physiology is limited. This study aimed to examine the physiological importance of AF-EVs in fetal development from the second trimester to term gestation. We obtained AF samples from routine second-trimester amniocentesis and prelabour Caesarean section at term. We isolated EVs using a combination of differential centrifugation, filtration, and ultracentrifugation and characterised them using nanoparticle tracking analysis, cryo-electron microscopy, and Western blotting. The differential EV proteome was analysed using label-free proteomics. We assessed the second trimester and term AF-EV properties through an enrichment analysis. The EV size and protein enrichment difference revealed a gestational-age-dependent variation in the predominant EV subtype. Second-trimester-derived EVs were enriched in ectosomes, while term EVs contained a significant proportion of exosomes. We identified several morphologies of AF-EVs, including unilamellar, multilamellar, multicompartmental and granular-centered EVs, across gestations. Proteomics analysis of AF-EVs identified 4137 proteins with high confidence, of which 1099 exhibited significant differential expression between the two groups. Second-trimester-enriched AF-EV proteins represented molecule assembly processes, metabolism and organogenesis. At term, AF-EV proteins corresponded to impending newborn functions such as immunity and digestion. In conclusion, we provide compelling evidence that EV biogenesis and secretion in the fetoplacental unit undergo significant alterations across gestation, revealing a complex and dynamic physiology and intercellular communication that adapts to the needs of the developing fetus.