Ulk1(S555) inhibition alters nutrient stress response by prioritizing amino acid metabolism
Ulk1(S555) inhibition alters nutrient stress response by prioritizing amino acid metabolism
Willoughby, O. S.; Nichenko, A. S.; Brisendine, M. H.; Amiri, N.; Henry, S. N.; Braxton, D. S.; Brown, J. R.; Specht, K. S.; Addington, A. K.; Zaitsev, A. V.; Burrows, S. T.; McMillan, R. P.; Zhang, H.; Tye, S. A.; Najt, C. P.; Craige, S. E.; Rhoads, T. W.; Warren, J. S.; Drake, J. C.
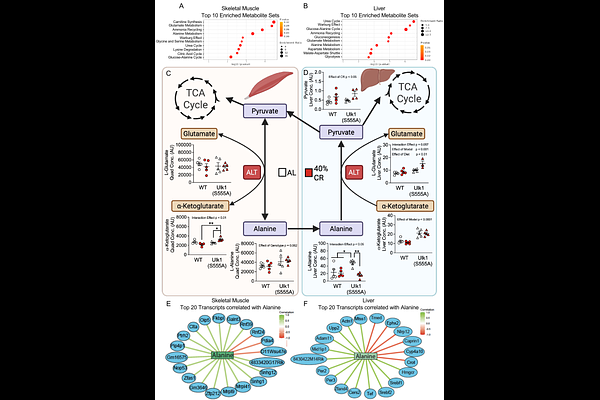
AbstractMetabolic flexibility, the capacity to adapt fuel utilization in response to nutrient availability, is essential for maintaining energy homeostasis and preventing metabolic disease. Here, we investigate the role of Ulk1 phosphorylation at serine 555 (S555), a site regulated by AMPK, in coordinating metabolic switching following short-term caloric restriction and fasting. Using Ulk1(S555A) global knock-in mice, we show loss of S555 phosphorylation impairs glucose oxidation in skeletal muscle and liver during short-term CR, despite improved glucose tolerance. Metabolomic, transcriptomic, and mitochondrial respiration analyses reveal a compensatory reliance on glucogenic amino acids, particularly alanine and serine, in Ulk1(S555A) mice, with sustained amino acid oxidation during fasting and blunted mitochondrial response to energetic stress. These findings establish Ulk1(S555) phosphorylation as a critical regulatory event linking nutrient stress to substrate switching and highlights an underappreciated role of Ulk1 in maintaining metabolic flexibility.