Click Chemistry Enables Rapid Development of Potent sEH PROTACs Using a Direct-to-Biology Approach
Click Chemistry Enables Rapid Development of Potent sEH PROTACs Using a Direct-to-Biology Approach
Schoenfeld, J.; Liebisch, N.; Brunst, S.; Weizel, L.; Knapp, S.; Kannt, A.; Proschak, E.; Hiesinger, K.
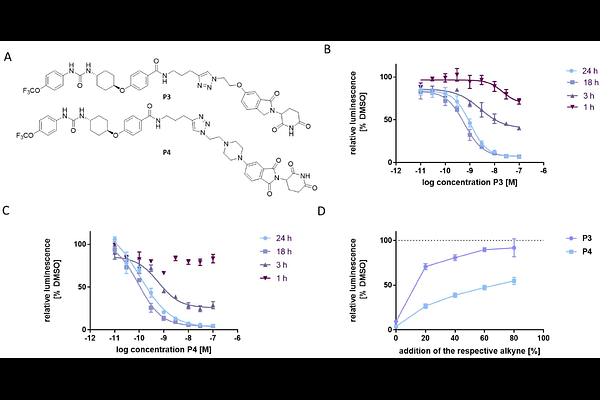
AbstractThe Direct-to-Biology (D2B) approach enables biological screening of crude reaction mixtures, eliminating the need for purification steps and thereby accelerating drug discovery. In this study, we developed a miniaturized D2B platform for the rapid synthesis of PROTAC degraders of soluble epoxide hydrolase (sEH). We used the copper-catalyzed azide-alkyne cycloaddition and optimized the conditions for 384-well PCR plate applications with 10 L reaction volumes on a 300 nmol scale. This approach enabled the D2B synthesis of 92 crude PROTACs from azide-functionalized CRBN-ligands and alkyne-linked sEH inhibitors. Biological screening using a HiBiT lytic degradation assay identified two hits which were resynthesized and exhibited subnanomolar DC50; values and degradation efficacy (Dmax). Thus, we established a scalable, cost-effective and time-saving D2B platform for the discovery of PROTACs in very small quantities. This methodology is particularly suitable for early-stage screening and hit validation assessing the degradability of a target.