Mutations outside the MR1 antigen binding groove differentially inhibit presentation of exogenous antigens
Mutations outside the MR1 antigen binding groove differentially inhibit presentation of exogenous antigens
Kulicke, C. A.; Lemon, C.; Krawic, J. R.; Ramirez, L. M. N.; Kim, S.-J.; Narayanan, G. A.; Tafesse, F. G.; Hildebrand, W. H.; Dobos, K. M.; Lewinsohn, D. M.
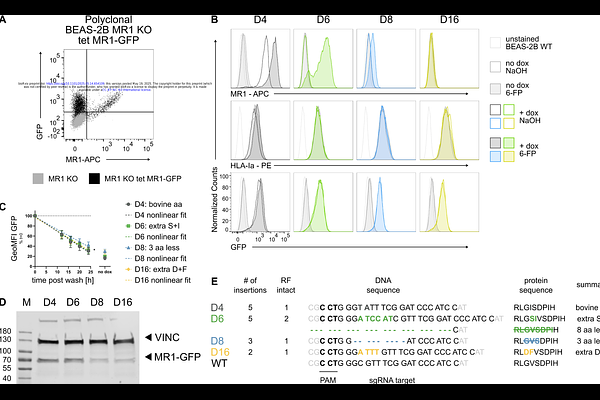
AbstractThe antigen presenting molecule MHC class I-related protein 1 (MR1) binds small molecule metabolites derived from microbial riboflavin biosynthetic pathways and presents them at the cell surface for surveillance by MR1-restricted mucosal-associated invariant T cells (MAIT cells). MR1 ligands can originate in the extracellular space or in endosomal compartments that contain microbial pathogens. Distinct, complementary antigen processing and presentation pathways enable MR1 to survey diverse intracellular locations and present both exogenous and intracellular antigens. Here, we generated a panel of BEAS-2B MR1 KO cells reconstituted with MR1 proteins mutated at amino acids 9-16. The mutated MR1 molecules differentially translocated to the cell surface in response to 6-formylpterin and differed in their ability to present mycobacterial antigens to MAIT cell clones. While they barely presented Mycobacterium smegmatis supernatant and other exogenous MAIT cell antigens, their ability to present antigens derived from mycobacterial infection and a 5-A-RU prodrug requiring endosomal processing remained largely intact. Protein co-immunoprecipitation and mass spectrometry-based proteomic analysis showed that mutated MR1 differentially associated with calnexin and {beta}2-microglobulin (B2M). Knock-down of B2M in cells over-expressing MR1 phenocopied the loss of exogenous antigen presentation but did not impact presentation of intracellular antigens. Thus, the MR1-mediated presentation of exogenous antigen appears to be limited by binding to B2M whereas the lower sensitivity to B2M deficiency implies that MAIT cell activation via the endosomal antigen presentation pathway may be limited by the availability of MR1 itself.