A novel host-encoded protein, ETP2, plays an important role in endosymbiont division in the trypanosomatid Angomonas deanei
A novel host-encoded protein, ETP2, plays an important role in endosymbiont division in the trypanosomatid Angomonas deanei
Maurya, A. K.; Cadena, L. R.; Ehret, G.; Nowack, E. C. M.
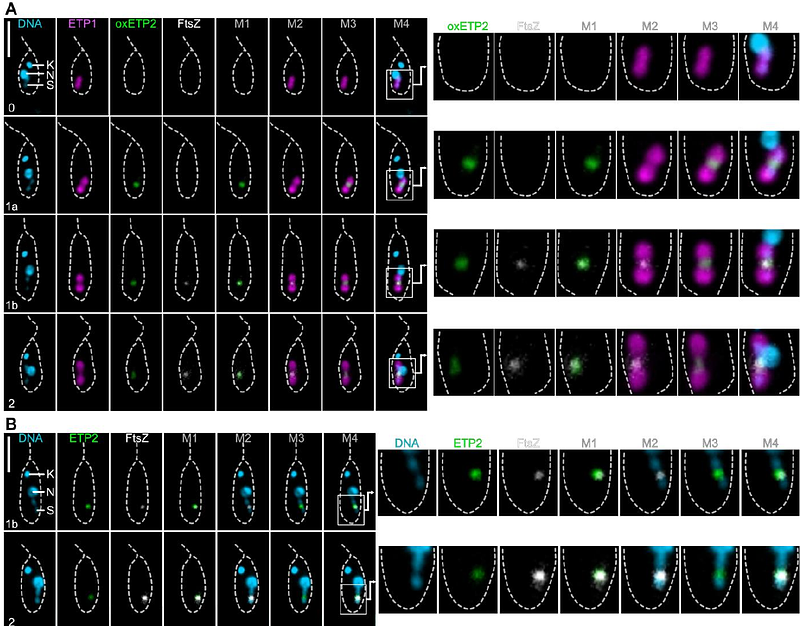
AbstractA single {beta}-proteobacterial endosymbiont, Candidatus Kinetoplastibacterium crithidii, resides in the cytosol of the trypanosomatid Angomonas deanei, and divides at a defined stage of its host\'s cell cycle. This endosymbiont has a highly reduced genome of 0.8 Mb and, notably, has lost most essential genes involved in bacterial cell division, resulting in a loss of division autonomy. It has been previously demonstrated that a host-encoded dynamin-like protein, ETP9, plays an indispensable role in the division of the endosymbiont. In this study, we identified a second nucleus-encoded component of the endosymbiont division machinery, termed ETP2, currently annotated as a hypothetical protein. We observed that ETP2 localizes in a cell cycle-dependent manner at the bacterial division site, alongside the bacterium-encoded FtsZ. Furthermore, we demonstrated that ETP2 plays an important role for timely endosymbiont division and segregation, as a large fraction of cells in an etp2 deletion mutant cell line exhibited either long, filamentous endosymbionts accompanied by severely distorted host cells or host daughter cells lacking endosymbionts. In silico analyses revealed that ETP2 is found exclusively in endosymbiont-harboring trypanosomatids and is most likely an intrinsically disordered protein. Collectively, our data suggests that ETP2, alongside the previously described ETP9, is an integral component of the endosymbiont division machinery. This finding highlights the evolution of a complex host-derived molecular mechanism that exerts tight control over its endosymbiont without requiring gene transfers from the bacterium.