Phosphoribosyl ubiquitination of SNARE proteins regulate autophagy in Legionella infection
Phosphoribosyl ubiquitination of SNARE proteins regulate autophagy in Legionella infection
Mukherjee, R.; Bhattacharya, A.; Tomaskovic, I.; Mello-Vieira, J.; Brunstein, M. E.; Basoglu, M.; Veenendaal, T.; Bailey, H.; Colby, T.; Misra, M.; Eimer, S.; Klumperman, J.; Munch, C.; Matic, I.; Dikic, I.
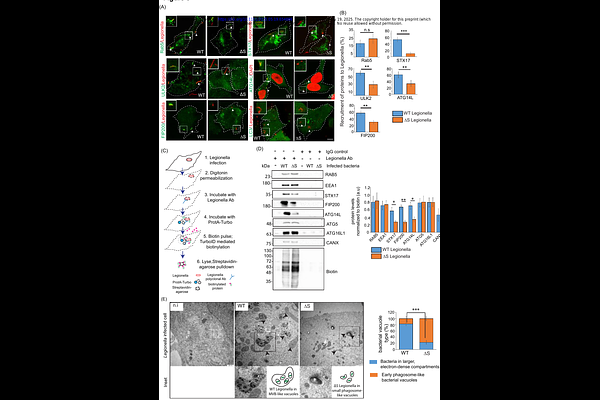
AbstractLegionella pneumophila is an intracellular pathogen that causes Legionnaires disease. The bacteria releases effector proteins some of which remodel the host autophagic-lysosomal pathways. One effector is RavZ, which delipidates ATG8 proteins, making Legionella-infected cells deficient in autophagy. Here we show that SidE effectors mediate the phosphoribosyl ubiquitination (PR-Ub) of autophagic SNARE proteins STX17 and SNAP29. STX17 modification induces recruitment of STX17+ membranes from the endoplasmic reticulum to Legionella-containing phagosomes to form replicative vacuoles. Using proximity labeling, biochemistry and Legionella infection studies, we have discovered a mechanism by which autophagy is hijacked by bacteria to recruit ER membranes to the bacterial vacuole which has autophagy markers but do not fuse with lysosomes. Mass spectrometric identification of PR-Ub sites and mutational studies show that PR-Ub modification of STX17 alters its interaction with ATG14L, which drives the recruitment of ER membranes to the bacterial vacuole in a PI3K dependent manner. On the other hand, PR-Ub of SNAP29 inhibits the formation of the autophagosomal SNARE complex (STX17-SNAP29-VAMP8) by steric hindrance, thereby preventing the fusion of bacterial vacuoles with lysosomes.