Impact of serum versus anticoagulant-containing plasma on influenza virusneuraminidase-based serological assays
Impact of serum versus anticoagulant-containing plasma on influenza virusneuraminidase-based serological assays
Do, T.; Kent, S. J.; Wheatley, A. K.; Koutsakos, M.
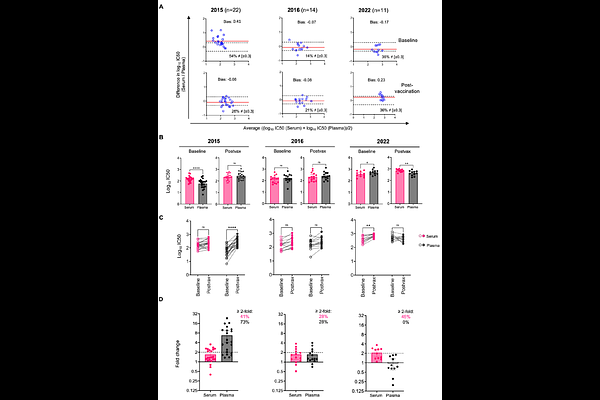
AbstractThe influenza virus neuraminidase (NA) is a promising target for next-generation influenza vaccines but standardized protocols for NA-based serological assays are lacking. Previous studies have demonstrated discordant results from haemagglutination inhibition and live virus microneutralization assays when comparing matched serum and plasma samples. It is therefore important to consider is the choice of serum or plasma samples in assays measuring influenza virus NA-specific antibodies. Here, we compared antibody titres against influenza A and B virus NAs in matched serum and different types of plasma using an enzyme-linked lectin assay (ELLA) and an enzyme-linked immunosorbent assay (ELISA). We observed good correlations between titres determined in serum and different types of plasma. However, there was variable and often poor agreement in the nominal titre values obtained from serum and different kinds of plasma in both ELLA and ELISA, with plasma samples often resulting in lower titres compared to serum samples. We also found differences in NA-specific responses to seasonal influenza vaccination assessed in serum versus plasma. Overall, our data demonstrate discrepancies between NA-specific antibody measurements in serum and plasma. We recommend the consistent use of serum as an important part of standardising NA-based serological assays.