Condensation of the RNA chaperone Hfq is coupled to inhibition of carbon assimilation and contributes to the stabilisation of regulatory RNAs in nitrogen starved Escherichia coli
Condensation of the RNA chaperone Hfq is coupled to inhibition of carbon assimilation and contributes to the stabilisation of regulatory RNAs in nitrogen starved Escherichia coli
McQuail, J.; Ellis, H.; Behrends, V.; Nadal, C.; Bischler, T.; Graefenhan, T.; Wigneshweraraj, R.
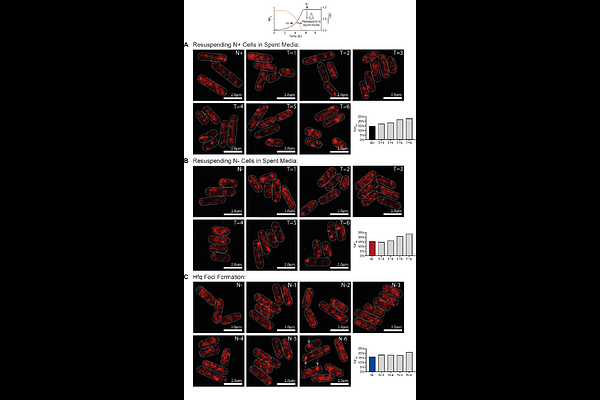
AbstractRibonucleoprotein-condensates are membraneless compartments that concentrate RNA-binding proteins and RNA and play key roles in cellular adaptation across both eukaryotes and bacteria. While the biological roles of ribonucleoprotein-condensates are better understood in eukaryotic systems, the knowledge of metabolic processes that govern their formation and their contribution to stress adaptation remains at a nascent stage in bacterial RNA biology. Hfq is an RNA-chaperone conserved in many bacteria that undergoes condensation in response to diverse stresses. Using nitrogen (N) starvation in Escherichia coli as a model stress condition, we show that Hfq condensation occurs independently of any extracellular cues, cytoplasmic shrinkage that cells undergo during N starvation or the canonical NtrBC-dependent adaptive response to N starvation. However, we demonstrate that Hfq condensation is coupled to the inhibition of carbon assimilation in N-starved E. coli. Further, by comparing the transcriptomes of wild-type bacteria and bacteria unable to form Hfq-condensates, we reveal that Hfq-condensates contribute to the stabilisation of Hfq-associated non-coding regulatory RNAs. We propose that coordination of carbon and N metabolism during N starvation, critical for metabolic adaptation, is accompanied by preservation of non-coding regulatory RNAs via Hfq condensation.