M. tuberculosis meets European Lead Factory - identification and structural characterization of novel Rv0183 inhibitors using X-ray crystallography
M. tuberculosis meets European Lead Factory - identification and structural characterization of novel Rv0183 inhibitors using X-ray crystallography
Riegler-Berket, L.; Goedl, L.; Polidori, N.; Aschauer, P.; Grininger, C.; Prosser, G.; Lichtenegger, J.; Sagmeister, T.; Parigger, L.; Gruber, C. C.; Reiling, N.; Oberer, M.
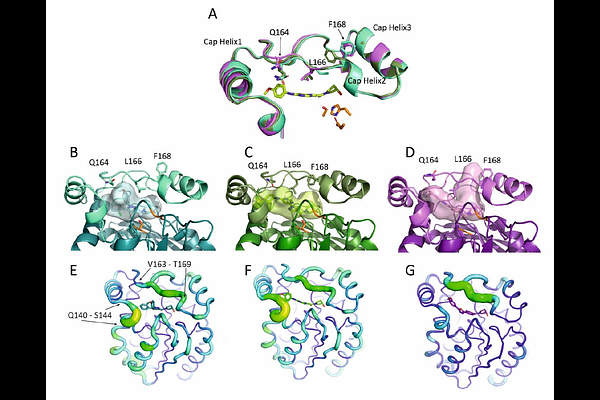
AbstractTuberculosis, caused by Mycobacterium tuberculosis (Mtb), remains a leading cause of mortality worldwide. Proteins involved in lipid metabolism, such as the monoacylglycerol lipase Rv0183, play critical roles during both the active and dormant phases of Mtb and present novel targets for therapeutic intervention. Through high-throughput screening at the European Lead Factory, we identified a novel chemotype characterized by a hydroxypyrrolidine ring, which demonstrated potent inhibition of Rv0183 and promising results in whole cell bacterial studies. Subsequent co-crystallization studies of this chemotype with Rv0183 revealed non-covalent interactions within the lipase's binding pocket, elucidating the inhibitory mechanism. Comparative analysis, augmented by AI-driven 3D-point-cloud approaches, distinguished Rv0183's ligand-binding cavity from that of human monoacylglycerol lipase, implying the possibility for species-selective inhibition. This selectivity was further supported by molecular docking simulations which validated the experimental binding affinities and predicted strong, specific binding modes. Our study presents not only the structural basis for the inhibition of Rv0183 by these novel hydroxypyrrolidine-based inhibitors but also demonstrates the utility of integrating computational and empirical methods to achieve species-specific targeting. This approach could minimize off-target effects in humans, marking a significant step toward developing more effective antitubercular therapies. The potential to selectively inhibit Mtb in its dormant state could lead to treatments that prevent the persistence and resurgence of the disease, addressing a crucial gap in the fight against tuberculosis.