Rational Design of Immunogenic Nanoparticles as a Platform for Enhanced Ovarian Cancer Immunotherapy in Mice
Rational Design of Immunogenic Nanoparticles as a Platform for Enhanced Ovarian Cancer Immunotherapy in Mice
Tang, L.; Marwedel, B.; Dang, C.; Olewine, M.; Jun, M.; Naydenkov, P.; Medina, L. Y.; Gayoso, V.; Doan, N.; OLeary, S. L.; Schiavone, C.; Cave, J.; Howard, T.; Watt, J. D.; Dogra, P.; Serda, R. E.; Noureddine, A.
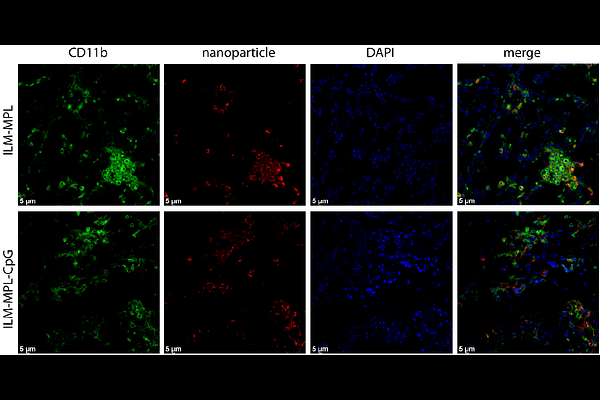
AbstractOvarian cancer immunotherapy remains a challenge based on the cold tumor microenvironment. Herein we present a rational design to create immunogenic nanoparticles as a multi-agent platform that promotes immune response in a mouse model of ovarian cancer. The hybrid lipid-silica nanosystem is capable of co-loading four types of cargo molecules including a model antigen, nucleic acid-based adjuvant Cytosine-p-linked to Guanine (CpG, TLR3/9 agonist), lipid-based adjuvant (MPLA, TLR4 agonist) integrated into the lipid coat, and optionally a small molecule drug, such as the chemotherapeutic agent oxaliplatin, a well-established treatment for ovarian cancer. The optimization of the nanoplatform in terms of lipid composition, functionalized silica dendritic core formation, and final charge, as well as their compatibility with the complex loading profile highlights an opportunity for enhanced survival of mice with advanced ovarian cancer compared to monotherapy. Furthermore, intraperitoneal administration led to preferential accumulation within tumor-burdened tissues with selective accumulation in myeloid cells. High myeloid cell cytotoxicity negated the benefits of oxaliplatin. The inclusion of CpG in the nanoparticle formulation enhanced the survival of mice with ovarian cancer. To interpret these outcomes and guide future design, we also developed a mathematical model of nanoparticle-driven immune activation, which quantified treatment efficacy and identified key parameters governing tumor response. The presented hybrid nanoparticle is tunable, enabling delivery of alternative molecules therefore, thereby highlighting a promising platform for the treatment of peritoneal cancers.