Gut Microbiome-derived Propionate Reprograms Alveolar MacrophagesMetabolically and Regulates Lung Injury Responses
Gut Microbiome-derived Propionate Reprograms Alveolar MacrophagesMetabolically and Regulates Lung Injury Responses
Maruyama, D.; Doan, T. N. M.; Tian, X.; Liao, W.-I.; Chaki, T.; Matthay, M. A.; Prakash, A.
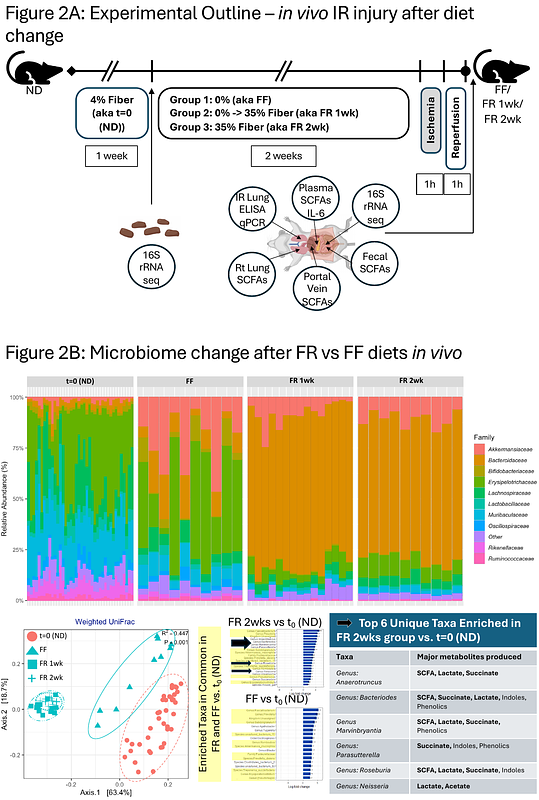
AbstractResponses to lung injury can vary between individuals and the diet and gut microbiome represent two underappreciated sources of inter-individual variability. The gut microbiome can influence lung injury outcomes through the gut-lung axis, but exactly how diet and its effects on the microbiota are involved remains unclear. We hypothesized that a dietary fiber intervention would enrich for the presence of short-chain fatty acid (SCFA)-producing fermentative bacteria in the gut microbiome, thereby influencing resting lung immunometabolic tone and influence downstream immune responses to lung injury and infection. To test this hypothesis, we fed mice fiber-rich (FR) and fiber-free (FF) diets and observed changes in the steady-state transcriptional programming of alveolar macrophages (AMs) in the absence of injury. Next, we examined the effects of FR and FF diets on the responses to sterile and infectious lung injury in vivo while simultaneously profiling gut microbiota and SCFA levels transmitted along the gut-lung axis. Finally, we validated our in vivo observations with mechanistic studies of the metabolic, signaling, and chromatin-modifying effects of specific SCFAs on lung AMs ex vivo and in vitro. Overall, a FR diet reprogrammed AMs and attenuated lung inflammation after sterile injury while exacerbating lung infection. This effect of FR diets could be transferred to germ-free (GF) mice by fecal microbiome transplantation (FMT) and depended on the ability of the microbiota to produce propionate. Mechanistically, SCFAs altered the metabolic programming of AMs and lung tissue ex vivo without a role for free fatty acid receptors (FFAR) or chromatin remodeling. These findings demonstrate that the gut-lung axis can regulate the resting metabolic tone through dietary fiber intake and the enrichment of SCFA-producing gut bacteria, as well as influence sterile and non-sterile lung injury responses through the activity of gut metabolites. These results provide further evidence to support the development of therapeutic dietary interventions or the use of specific gut metabolites to preserve or enhance specific aspects of host pulmonary immunity.