Molecular dynamics simulations reveal a mechanism of calcium homeostasis driving homotrimerisation and heterotrimerization of type I collagen
Molecular dynamics simulations reveal a mechanism of calcium homeostasis driving homotrimerisation and heterotrimerization of type I collagen
Johnson, E.; de Souza, J. V.; Bronowska, A. K.; Laird, E. G.
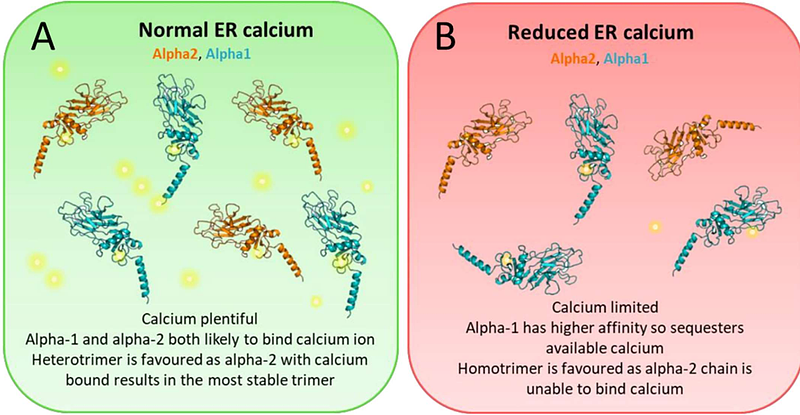
AbstractType I collagen is the main structural protein of vertebrates and forms molecular trimers from the COL1A1 and COL1A2 gene products; pro-alpha-1(I) and pro-alpha-2(I)), during biosynthesis. The amino acid sequence of the C-propeptide of collagen, which is removed before collagen fibril formation, initially drives heterotrimerisation and calcium ions are required for trimers to form. The homotrimeric form is associated with age-related diseases including cancer, fibrosis, musculoskeletal and cardiovascular conditions but circumstances under which the abnormal homotrimer may form were poorly understood. Here we used molecular dynamics simulations of the C-propeptide protein structure, to show that intra- and intra-chain hydrogen bonding is affected by the loss of calcium and that chains become destablised, particularly at the interfaces of each chain. Loss of calcium resulted in an increased distances between cysteine residues that form inter-chain disulphide bonds, predicting an inability for disulphides to form in the absence of calcium. Pulling simulations and modeling calcium dissociation from monomers showed that calcium ions were more strongly bound to the alpha-1(I) than the alpha-2(I) chain. However, pulling a single alpha chain from the heterotrimer or homotrimer demonstrated that the alpha-2(I) chain had a higher affinity to trimers than a third alpha-1(I) chain. Hence although heterotrimerisation is normally favoured, in reduced calcium conditions the homotrimer can form by sequestering available calcium to the alpha-1(I) chains. This study provides a molecular explanation for a calcium-based mechanism driving heterotrimerisation versus homotrimerisation of type I collagen.