Inhibition of TBK1/IKKe mediated RIPK1 phosphorylation sensitizes tumors to immune cell killing
Inhibition of TBK1/IKKe mediated RIPK1 phosphorylation sensitizes tumors to immune cell killing
Piskopou, A.; Vredevoogd, D. W.; Kong, X.; Altelaar, M.; Peeper, D. S.; Stecker, K. E.
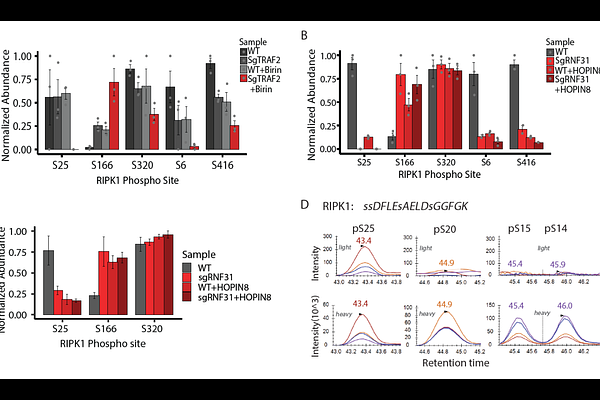
AbstractResistance to immune cell-mediated cytotoxicity poses a significant challenge in cancer therapy, compromising the efficacy of immunotherapeutic approaches such as immune checkpoint blockade (ICB) treatment. To enhance therapy outcomes, it is crucial to identify interventions that can synergize with ICB therapy to overcome tumor resistance. Therefore, we need to define the cellular mechanisms that sensitize tumors to cytotoxic T cells. CD8 T cells rely on cytokines such as TNF to carry out their cytotoxicity against tumors, and recent findings link select tumor mutations in the TNF pathway to increased T cell killing, in a manner dependent on RIPK1 kinase. Here, we demonstrate that sensitized tumor cells fail to initiate inhibitory RIPK1 phosphorylation at site S25 upon T cell attack, thereby foregoing a pro-survival checkpoint early in TNF signal transduction. Consequently, tumor cells experiencing a loss of TNF-induced RIPK1 S25 phosphorylation exhibit increased RIPK1 activation and fail to recruit non-canonical IKK kinases (TBK1 and IKKe) to the TNFR1 complex. Functional knockouts of TBK1 and IKKe in melanoma cells result in heightened sensitivity not only in CD8 T cell but also in Natural Killer cell attacks. Our findings indicate that preventing TBK1 and IKKe recruitment to the TNF signaling complex, thereby blocking RIPK1 pro-survival phosphorylation and promoting direct RIPK1 activation, is a tractable strategy to increase tumor sensitivity to immune cell killing and has the potential to benefit current immunotherapy interventions.