Molecular insights into inhibitor action on a key bacterial metabolic enzyme Cystathionine β-Synthase
Molecular insights into inhibitor action on a key bacterial metabolic enzyme Cystathionine β-Synthase
Polepalli, S.; Roy, A.; Mondal, B.; Singh, A.; Dutta, S.
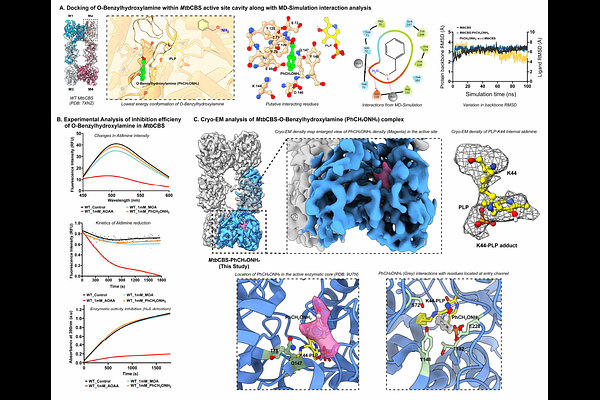
AbstractTuberculosis (TB) remains a major global health threat, with Mycobacterium tuberculosis (Mtb) infecting nearly a quarter of the global population. Drug-resistant TB and HIV-TB co-infections emphasize the need for novel therapeutic approaches targeting essential metabolic pathways. Here, we investigated Mtb cystathionine {beta}-synthase (MtbCBS), a PLP-dependent enzyme critical for sulfur metabolism and redox regulation, owing its potential as a therapeutic target. We present the first high-resolution cryo-EM structure of MtbCBS bound to aminooxyacetic acid (AOAA), and employed molecular dynamics (MD) simulations, quantum mechanics/molecular mechanics (QM/MM) calculations, and comparative inhibition studies to reveal the molecular basis and determinants governing irreversible PLP-enzyme inhibition. Our Cryo-EM structural analysis revealed two highly conserved active-site residues, T75 and Q147, critically stabilizing the inhibitor complex. Through molecular mimic studies, we demonstrated the precise structural factors and electronic features critical for inhibition efficiency. These findings provide the first mechanistic rationale for PLP enzyme inhibition and offer a generalizable framework for designing covalent inhibitors targeting PLP-dependent enzymes implicated in infectious diseases, cancer, and neurological disorders.