Dynamic acetylation of a conserved lysine impacts glycerol kinase activity and abundance in the haloarchaeon Haloferax volcanii
Dynamic acetylation of a conserved lysine impacts glycerol kinase activity and abundance in the haloarchaeon Haloferax volcanii
Sanchez, K. M.; Addagarla, M.; Judd, H. N.; Wang, X.; Maupin-Furlow, J. A.
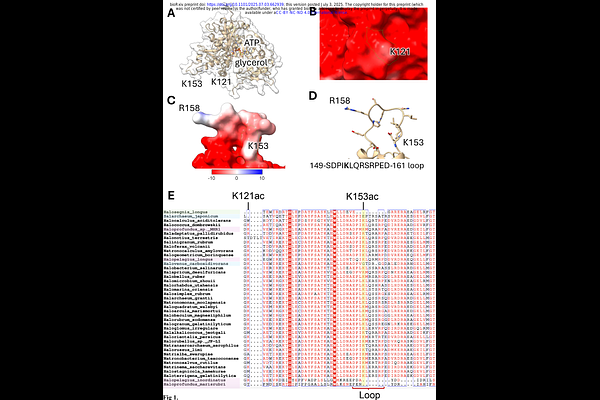
AbstractHaloferax volcanii is a halophilic archaeon that preferentially utilizes glycerol as a carbon source, placing glycerol kinase (GK, glpK) at the center of its metabolism. In contrast to bacterial GKs, which are often regulated by allosteric inhibition, H. volcanii GK lacks this mode of control, indicating alternative regulatory mechanisms. Here, we show that lysine acetylation of H. volcanii GK enhances its activity and abundance during growth on glycerol, with K153 identified as the primary site of modification. Structural modeling and comparative genomics revealed that K153 resides in a conserved flexible loop common to haloarchaeal GKs. Carbon shifts from glucose to glycerol led to increased activity and enrichment of the K153-acetylated form, as determined by AQUA-MS. GK and the acetylation mimic K153Q supported growth on glycerol, while the non-acetylatable K153R variant did not. Thermal shift analysis showed that the K153R substitution reduced GK stability, while K153Q had no effect. Size exclusion chromatography indicated that GK is predominantly dimeric but forms a tetramer when purified from glycerol-grown cells and assayed with glycerol - coinciding with the highest K153 acetylation levels. Kinetic analysis revealed that K153 acetylation is required to maintain cooperative substrate binding, with the non-acetylatable K153R variant exhibiting a loss of allosteric behavior. The GNAT-family acetyltransferase Pat2 was found to acetylate GK at K153, and{Delta} pat2 mutants exhibited reduced GK protein abundance, linking Pat2 to regulation of GK. These results identify a dynamic, carbon source-responsive lysine acetylation mechanism that modulates GK, highlighting lysine acetylation as a key component of haloarchaeal metabolic regulation.