Rankings of tuberculosis antibiotic treatment regimens are sensitive to spatial scale, detection limit, and initial host bacterial burden
Rankings of tuberculosis antibiotic treatment regimens are sensitive to spatial scale, detection limit, and initial host bacterial burden
Michael, C. T.; Budak, M.; Maiello, P.; Kracinovsky, K.; Rodgers, M.; Tomko, J.; Lin, P. L.; Flynn, J. L.; Linderman, J. J.; Kirschner, D.
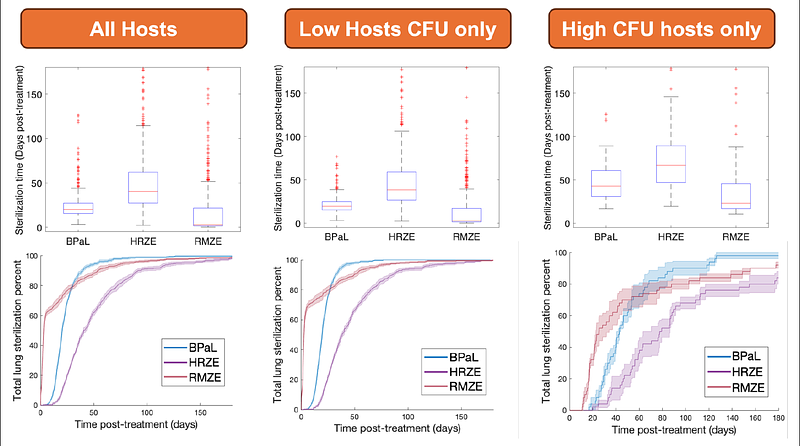
AbstractPulmonary infection after inhalation of Mycobacterium tuberculosis (Mtb) causes tuberculosis (TB). In humans and non-human primates, TB presents with lung granulomas - complex spheroidal structures composed of immune cells and bacteria. These granulomas often have centralized caseum (necrotic tissue) where mycobacteria are spatially quarantined, complicating antibiotic treatment. It is challenging to untangle which of the many host-pathogen interactions drive the heterogeneous infection and treatment outcomes observed at between-host and within-host scales. Treatment of TB involves administration of multiple antibiotics for extended periods of time. Determining which antibiotic regimens are optimal for reducing treatment times and toxicity is a goal of recent TB eradication campaigns. TB treatment clinical trials with multiple different drug regimens are expensive and challenging. Here, to determine responses to various combinations of antibiotics, we simulate antibiotic treatments in HostSim, our whole-host mechanistic, multi-scale computational model of Mtb-infection within primates. HostSim tracks the dynamics of pulmonary Mtb-infection within a primate host over molecular, cellular, tissue, organ, and whole-host scales. We also create a heterogenous virtual cohort, comprising distinct hosts, for virtual clinical trials. Virtual drug treatments are represented by a newly integrated pharmacokinetics / pharmacodynamics and drug-interaction model. We simulate treatment with regimens containing up to four commonly-prescribed TB antibiotics (e.g., HRZE or BPaL). Our approach allows us to identify both (1) which hosts/granulomas most improved with each treatment, and (2) which mechanisms most influence outcome heterogeneity. HostSim recapitulates several mechanisms known to correlate with total bacterial burden (CFU) of both hosts and granulomas such as volume of caseum and bactericidal activity of T cells and macrophages. By tracking experimental and clinical measurements in silico, we virtually recreate several drug rankings from literature. Strikingly, we find that many ranking methods of overall antibiotic regimen efficacy are strongly influenced by the \'definition of improvement\' used and, in some cases, the experimental detection threshold of CFU. Other rankings depend on initial bacterial burden of hosts and granulomas. Our work suggests that metrics for drug regimen optimality may be orthogonal, which may explain seemingly-contradictory findings from clinical and experimental studies.