MORC2 directs transcription-dependent CpG methylation of human LINE-1 transposons in early neurodevelopment
MORC2 directs transcription-dependent CpG methylation of human LINE-1 transposons in early neurodevelopment
Dorazehi, F.; Francesconi, C.; Pandiloski, N.; Castilla-Vallmanya, L.; Albecka, A.; Koutounidou, S.; Mohan, S. B.; Adami, A.; Katsikas, A.; Matijevic, J.; Karlsson, O.; Davis-Hansson, C.; Johansson, J. G.; Modis, Y.; Jakobsson, J.; Douse, C. H.
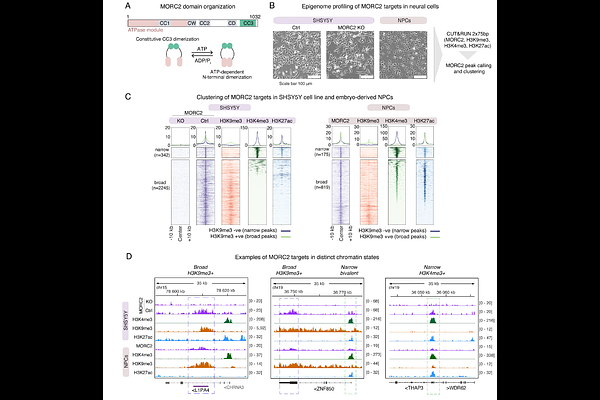
AbstractMethylation of CpG dinucleotides is essential for silencing genomic repeats such as LINE-1 retrotransposons (L1s) in the germline and soma. Evolutionarily-young L1s are transcribed in human pluripotent stem cells, but how CpG methylation is patterned to these L1s upon exit of pluripotency is unknown. Here we investigate the critical functions of chromatin regulator MORC2 in epigenome reprogramming of the repetitive genome in early human neurodevelopment. We find that reversible ATP-dependent dimerization is required for MORC2 accumulation over L1s but not gene promoters. Mutation of the MORC2 ATPase module, causative for neurodevelopmental disorders, severely disrupts the distribution of MORC2 chromatin binding, leading to simultaneous loss of L1 transcriptional control and hyper-repression of clustered ZNF genes in human pluripotent stem cells. Upon neural differentiation these phenotypes persist due to striking, targeted defects in CpG methylation patterning. Together our results define the vital role of MORC2 in safeguarding the somatic human genome upon exit of pluripotency by directing CpG methylation patterning over transcriptionally-active retrotransposons in a manner analogous to the piRNA pathway in the germline.