The mechanism of ribosomal recruitment during translation initiation on Type 2 IRESs
The mechanism of ribosomal recruitment during translation initiation on Type 2 IRESs
Bhattacharjee, S.; Abaeva, I.; Brown, Z.; Arhab, Y.; Fallah, H.; Hellen, C. U. T.; Frank, J.; Pestova, T.
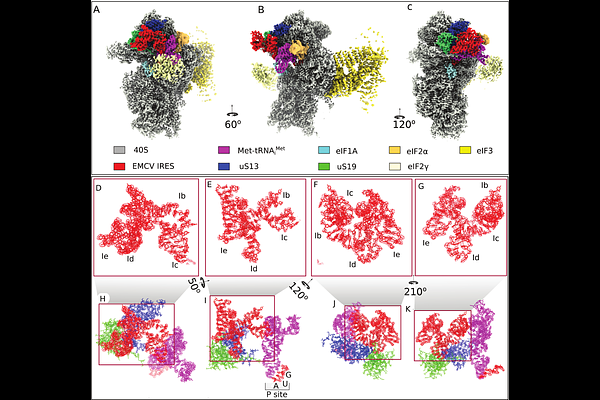
AbstractThe encephalomyocarditis virus (EMCV) IRES and other Type 2 IRESs comprise domains H-L and specifically interact with eIF4G/eIF4A through their essential JK domain. However, the JK domain is not sufficient for IRES function, which also requires the preceding domain I of unknown function. To identify interactions that drive ribosomal recruitment of eIF4G/eIF4A-bound Type 2 IRESs, we determined the cryo-EM structure of 48S initiation complexes formed on the EMCV IRES. It revealed that the apical domain I cloverleaf contacts ribosomal proteins uS13 and uS19 via its Id subdomain and that the essential GNRA tetraloop in subdomain Ic interacts directly with the T{psi}C domain of initiator tRNA. Functional assays supported the exceptional role of these interactions for initiation on this IRES. The strong conservation of primary and secondary structures of the apex of domain I among Type 2 IRESs suggests that the reported interactions are a common essential feature of them all.