Transient Poly(ADP-Ribose) Triggers FUS Condensation Hysteresis via a Prion-Like Mechanism
Transient Poly(ADP-Ribose) Triggers FUS Condensation Hysteresis via a Prion-Like Mechanism
Liu, H.; Cai, Y.; Shi, L.; Pillai, M.; Das, N.; Tarbox, H. E.; Ge, Y.; Yue, K.; Yang, X.; Rath, P.; Badiee, M.; Fabilane, C. S.; Spangler, J. B.; Bedford, M. T.; Myong, S.; Fried, S. D.; Ding, X.; Leung, A. K. L.
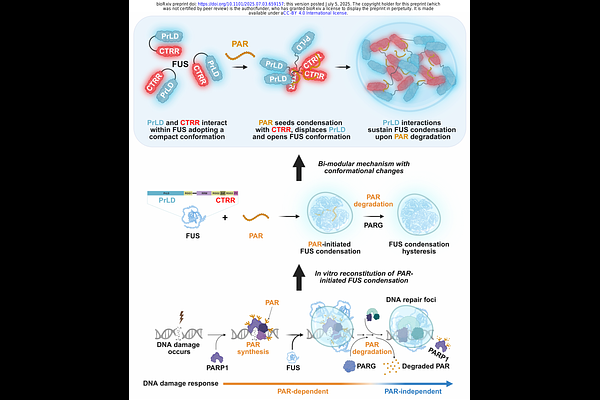
AbstractHysteresis--where a system retains memory of a transient stimulus--is common in signaling but can also arise in intracellular organization. DNA repair foci, a type of biomolecular condensate, are initiated by the short-lived noncanonical nucleic acid poly(ADP-ribose) (PAR). PAR recruits proteins with prion-like domains (PrLDs), such as Fused in Sarcoma (FUS), and initiates their condensation, which persists even after PAR degradation. How FUS transitions from PAR-dependent to PAR-independent condensation remains unclear. Here, we show that PAR binding triggers a conformational switch in FUS, enabling sustained condensation. PAR binds to the C-terminal arginine-rich region of FUS, displacing intramolecular contacts, and exposing the N-terminal PrLD. This conformational opening allows PrLD interactions in trans, stabilizing condensates independently of PAR. FUS thus undergoes a regulated, nucleated conformational conversion--reminiscent of classical prions. This mechanism implies a paradigm of nucleic acid-induced conformational memory that may underlie hysteresis in intracellular organization in health and disease.