HO-3867 mediated modulation of Extracellular vesicles and Tumor microenvironment: A novel immunotherapeutic strategy for ovarian cancer
HO-3867 mediated modulation of Extracellular vesicles and Tumor microenvironment: A novel immunotherapeutic strategy for ovarian cancer
Yadagiri, G.; Chakrapani, L. N.; Sundaram, S.; Velasquez, J.; Hughes, T.; Vendrell, G. S.; Bognar, B.; Wang, Q.-E.; O Malley, D. M.; Cohn, D. E.; Karuppaiyah, S.; Dorayappan, K. D. P.
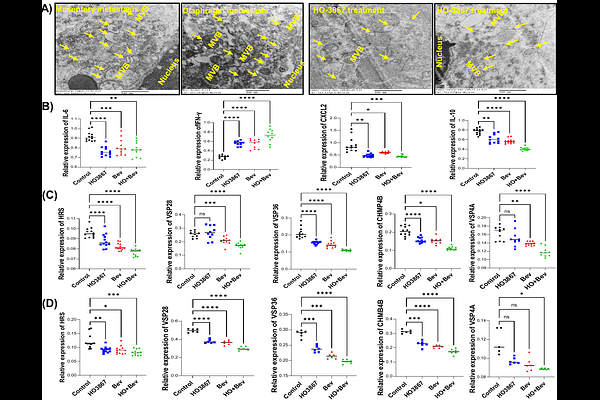
AbstractAbstract Introduction: Ovarian cancer (OC) remains the most lethal gynecologic malignancy, with poor long-term survival, largely due to its diagnosis at advanced stages and its high rate of recurrence with resistance to platinum therapies. This study evaluates the therapeutic efficacy of HO-3867, a STAT3 inhibitor, and bevacizumab, an anti-VEGF antibody, alone and in combination in an immunocompetent (IC) syngeneic ovarian cancer progression model. Methods: The ability of HO-3867 to induce macrophage polarization was assessed in RAW264.7 cells, while its impact on anti-tumor immunity was evaluated in splenocytes co-cultured with ID8 and OC ascites cells. Immune modulation, including cytokine induction, extracellular vesicle (EV) release, and endosomal sorting complex required for transport (ESCRT) protein expression, was analyzed in-vitro and using in-vivo OC model. The therapeutic effects of HO-3867 and bevacizumab were examined in vivo by measuring ascites volume, EV secretion levels, and immune cell populations via flow cytometry. Results: Our results demonstrate that combination therapy significantly reduced ascites accumulation, and limited peritoneal disease spread in ovarian cancer mouse models. EV analysis revealed a decrease in total EVs and CD9+ subpopulations, which correlated with the associated ESCRT proteins involved in EV formation and secretion implicating EVs in tumor progression and immune modulation. Flow cytometry analysis of ascites immune cell populations showed that combination therapy reduced myeloid-derived suppressor cells (MDSCs) and their PD-L1 expression, while enhancing CD8+ T cell cytotoxicity via granzyme B secretion. Cytokine profiling revealed upregulation of IFN-{gamma} and downregulation of IL-6, IL-10 and CXCL-2 suggesting enhanced anti-tumor immune response. Furthermore, HO-3867 promoted macrophage polarization toward tumoricidal M1 phenotype and reduced MDSC expansion in- vitro, enhancing anti-tumor immunity. Conclusion: HO-3867 and bevacizumab synergistically reprogram the tumor microenvironment, inhibit EV-mediated progression, and enhance antitumor immunity. These findings support its immunomodulatory potential in ovarian cancer ascites, offering a promising therapeutic strategy.