EWSR1::ETS-low cells promote metabolic reprogramming of the tryptophan-kynurenine-AHR axis, immunosuppression, and poor outcome in Ewing sarcoma
EWSR1::ETS-low cells promote metabolic reprogramming of the tryptophan-kynurenine-AHR axis, immunosuppression, and poor outcome in Ewing sarcoma
Carreno-Gonzalez, M. J.; Hanssen, K. M.; Sadik, A.; Henneberg, A.; Ehlers, A. C.; Ceranski, A. K.; Kołodynska, Z. A.; Zimmermann, M.; Imle, R.; Geyer, F. H.; Ritter, A.; Banito, A.; Knott, M. M. L.; Ohmura, S.; Cidre-Aranaz, F.; Opitz, C. A.; Grünewald, T. G. P.
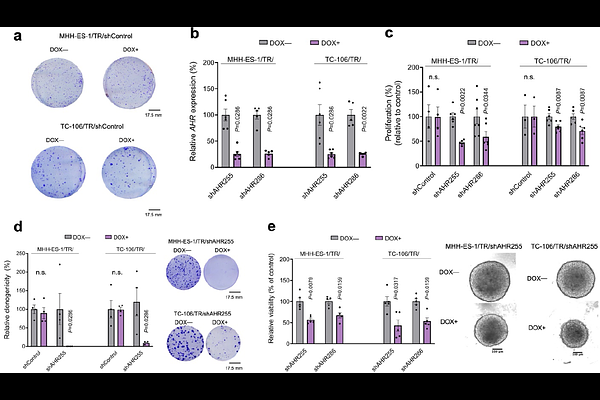
AbstractHow activational changes of driver oncogenes can promote metabolic reprogramming and cancer progression is largely uncharted territory. To address this issue, we employed Ewing sarcoma (EwS) as a model as it is driven by a single driver oncogene, namely chimeric EWSR1::ETS fusion transcription factors, whose fluctuating expression levels promote diverse transcriptional programs. While EWSR1::ETS-high cells display a rather sessile but proliferative phenotype and EWSR1::ETS-low cells are more invasive, the mechanisms underlying these different phenotypes remain poorly characterized. Here, using an integrative functional genomics approach, we show that low EWSR1::ETS fusion activity in patient tumors associates with poor outcome and increased signaling of the aryl hydrocarbon receptor (AHR). AHR signaling enrichment correlates with increased tumor infiltration by immunosuppressive cells and worse overall patient survival. Knockdown of EWSR1::ETS in EwS spheroid models cultured in human plasma-like media strongly alters tryptophan metabolism resulting in increased secretion of the AHR ligand kynurenine. Silencing of EWSR1::ETS reduced killing of EwS cells by natural killer (NK) cells, while AHR silencing increases NK cell-mediated killing. From a cell-intrinsic perspective, AHR knockdown inhibits proliferation, clonogenic, and spheroidal growth of EwS cells in vitro, which can be recapitulated by pharmacological AHR inhibition. Furthermore, AHR knockdown decreases tumor growth and metastasis in vivo. Collectively, our results illustrate how oncogene-mediated metabolic reprogramming of amino acid metabolism can translate into more aggressive disease phenotypes in cancer.